
Company and People Notes: Evotec and Renovis enter agreement, Amgen to lay off 675 workers, more.

Company and People Notes: Evotec and Renovis enter agreement, Amgen to lay off 675 workers, more.

The US Food and Drug Administration has denied shipments of active pharmaceutical ingredients manufactured at a production facility of Kunshan Chemical and Pharmaceutical Co. for violation of good manufacturing practices, according to an FDA warning letter issued Sept. 6, 2007.

Company and People Notes: Catalent expands Bolton, UK, warehouse; Biotica appoints Edward E. Hodgkin as CEO and director; more.

Pfizer plans to cease all remaining manufacturing operations at its facility in Sandwich, Kent, United Kingdom. The closure will result in the loss of approximately 420 jobs, phased over the next two years, according to a company release

Company and People Notes: Baxter and Halozyme Expand Relationship, Crucell Names COO, More.

More than 100 people attended this week’s Regulatory Affairs Conference by the International Pharmaceutical Excipients Council of the Americas to discuss the latest trends and challenges in the excipient supply chain.

Aptuit expands its drug-development capabilities with the formation of Aptuit Laurus to take advantage of the growing pharmaceutical outsourcing market in India.
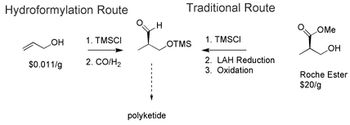
Researchers forward approaches for catalytic hydroformylation, asymmetric hydrogenation, and biocatalysis to achieve enantioselectivity.

Appendix: definitions and regulations, Federal Food, Drug, and Cosmetic Act; Appendix: definitions and regulations, Title 21 Code of Federal Regulations; Appendix: definitions and regulations, Compliance Policy Guides
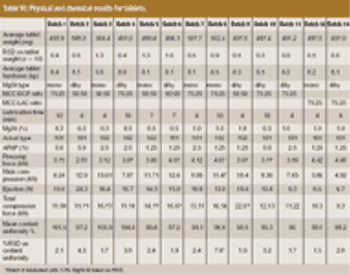
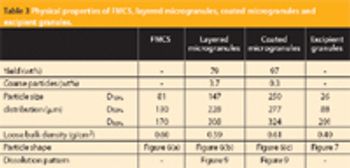
The influence of magnesium stearate (MgSt) on powder lubrication and finished solid-dose properties presents big challenges to drug manufacturers.

This article presents collaborative positions among excipient manufacturers, drug product manufacturers, and members of the US Pharmacopeia on key issues pertaining to the control of pharmaceutical excipients stemming from a recent Pharmaceutical Quality Research Institute workshop.

Fiera Milano Rho Exhibition Centre, Milan, Italy 2–4 October 2007
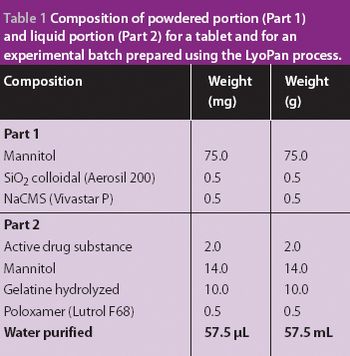
A new economical method for producing fast-melting lamina-like dosage forms.
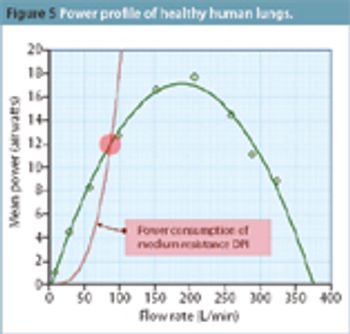
This article investigates how the industry can test inhalers in a way that is most representative of typical use.
This article describes how rapidly disintegrating tablets containing a large quantity of an intensely bitter drug were successfully developed with a suitable level of masking, tablet hardness, disintegration property, dissolution profile and mouth feel.

A new Raman spectroscopic method to detect magnesium stearate in powder blends and tablets is described. High-volume pharmaceutical manufacturing requires the use of lubricants to facilitate tablet ejection from compressing machines. However, lubricants may also bring a number of undesired problems that have been widely documented in pharmaceutical scientific literature. New analytical methods are needed to understand lubrication and provide process knowledge in support of FDA's process analytical technology initiative. The detection of magnesium stearate in lactose, mannitol, corn starch and other commercially important excipients is reported. The Raman spectroscopic method has a detection limit of about 0.1% (w/w) based on the 2848 cm-1 band that corresponds to the symmetric stretch of the methylene group in magnesium stearate.

The authors evaluate the scalability of foam-granulation technology using continuous foam addition in high-shear granulation equipment at the laboratory, pilot and manufacturing scales. Immediate- and controlled-release model formulations were used. Continuous and batch addition of foam were compared for the controlled-release model formulation at the manufacturing scale, and physical testing was performed on the granules and finished tablets.

As China emerges as a significant supplier of pharmaceutical ingredients, it must assure other countries of the safety of its excipients.

Identifying polymorphs is a crucial part of the drug-development process as researchers forward select methods to improve detection.

Contract manufacturers and pharmaceutical ingredient suppliers proceed with select investments in biologics manufacturing, small-molecule synthesis, and formulation as the industry prepares for CPhI Worldwide in Milan.
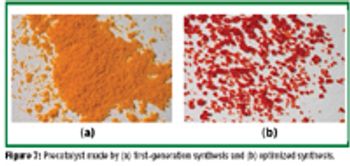
Single-enantiomer drugs represent an increasingly large share of new chemical entities, leading to approaches in asymmetric synthesis.

A spate of drugs are scheduled to come off patent, offering vast potential and competition.

The financial performance of the pharmaceutical majors was generally favorable through the first half of 2007, with most companies reporting moderate to double-digit growth. Industry leader Pfizer, however, reported a sales decline for the second quarter and flat revenues through the first half of 2007. Pfizer Chairman and CEO Jeffrey Kindler says the company remains committed to its plans for cost-cutting, more outsourcing, and increasing its position in biologics.
Catalytic routes to producing atorvastatin and sitagliptin are recent advancements.

Although North America accounts for the largest share of the pharmaceutical market, Brazil, China, India, Indonesia, Mexico, Russia, and Turkey are projected to account for almost one-fifth of the global market by 2020. The rising participation in select countries' drug-development activities is evident by recent investment and outsourcing by the pharmaceutical majors.