
Currently, high-production rates and continuous production processes favor existing tableting technologies. However, if tablet development becomes rate-limiting in the future, alternative technologies may prove attractive.

Currently, high-production rates and continuous production processes favor existing tableting technologies. However, if tablet development becomes rate-limiting in the future, alternative technologies may prove attractive.

Some of the common problems faced by formulators and how using ion exchange resins may be able to solve them are discussed.
In this series of articles, bin blender performance is comprehensively reviewed using both free-flowing and cohesive mixtures. In part 1, an introduction to tools and techniques is presented, followed by an examination of parameter effects, mixing mechanisms, and the effects of cohesion on mixing.

Bin blender performance is comprehensively reviewed using both free-flowing and cohesive mixtures.

The authors provide a combined source of density data and show that denity can be used as an equipment-independent sclaing parameter in the manufacture of common pharmaceutical solids.

Spreadability of a semisolid forrmulation is affected by factors such as formulation characteristics, rate and time of shear, temperature, site of application, and the presence of various additives.

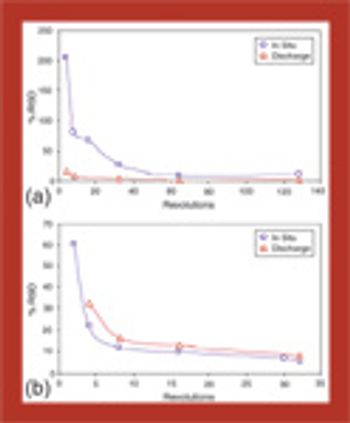
Dynamic powder-sampling methods may be the best techniques for obtaining a representative sample for determining particle-size distribution.

Powdered self-emulsified dosage forms provide an attractive alternative to filled-capsule preparations, but the selection of a proper excipient is crucial.

Semisolid dosage forms are advantageous in terms of their easy application, rapid formulation, and ability to topically deliver a wide variety of drug molecules.

Low levels of some microorganisms that are present in oral solid dosage forms are unlikely to present a risk to patients. The amount of water activity in these products can help determine when microbiological testing should be conducted.

To achieve the maximum performance from a tablet press run, operators must learn to distinguish between machine-related issues and granulation-related issues.

Tablet manufacturers often overlook critical basic concepts or established practices. The author draws from his own experiences and discusses how solid dosage manufacturers can improve production and product quality by optimizing major unit operations.

The authors modified a three-layer press to operate a compression-coated tablet process that may offer several advantages over tradditional compression coating operations.

The author assesses the compressibility of Plasdone S- 630 copovidone-based tablet formulations using roller-compaction and direct-compression processes.

Both academia and industry have been encouraged to generate quick­dissolving dosage forms as the demand for fast­disintegrating tablets and capsules has steadily increased.

Appropriate sampling procedures, proper preparation, and correct instrumental parameters may yield differing, but correct, results.

Evolution of an existing drug molecule from a conventional form to a novel delivery system can significantly improve its performance in terms of patient compliance, safety, and efficacy. These days,drug delivery companies are engaged in the development of multiple platform technologies to get competitive advantage, extend patent life, and increase market share of their products.