
Learn how to prevent common causes of product loss.

Learn how to prevent common causes of product loss.

Advances in equipment, instrument, and control systems are enabling online monitoring of continuous API manufacturing.

Although technical paths for continuous solid-dosage manufacturing have been laid out and equipment and control systems have been developed, industry is slow to move forward.

Anil Kane, executive director, Global Head of Formulation Sciences, Pharmaceutical Development Services at Patheon discusses key parameters in the development and manufacturing of oral solid-dosage forms.

Advances in process analytical technology have been achieved, but significant challenges remain.

A venture between GEA and Siemens aims to familiarize more pharmaceutical companies with more modern control and continuous processing.

This novel technology was developed in response to challenges involved in conventional manufacturing of multilayer tablets, including in-line control of the tablet weight, the tendency to delamination, direct contact between the two tablet layers, and cross contamination.

Conferences focused on continuous solid-dosage manufacturing aim to spread the word about technical capabilities and alleviate perceptions of regulatory uncertainty.

Capsule filling is a complex process, and the product to be encapsulated must be well developed to ensure mass uniformity.

Improved process analytical technology and new ways of thinking seek to enhance measurement and control for next-generation pharmaceutical manufacturing.

Soft sensors are powerful tools that can be used along with spectroscopic instruments in on-line measurement.

Camfil Air Pollution Control has expanded its testing laboratory and added a dust collection test rig for the ANSI dust collection standard.

GEA’s ConsiGma continuous tableting line combined with Siemens’ automation and Sipat data management systems enables continuous manufacturing.

FDA and BARDA awarded a contract to Continuus Pharmaceuticals to develop an end-to-end continuous manufacturing process for solid-dosage drugs.

Catalent adds two softgel facilities and packaging capabilities with acquisition of Canada-based Accucaps.

A lifecycle approach can be used to develop GMP-compliant cleaning procedures for continuous manufacturing of solid-dosage pharmaceuticals.

Flexible batch sizes for semi-continuous unit operations, such as tableting and encapsulation, can improve efficiency while maintaining quality.

PharmTech’s 2016 survey shows general satisfaction with existing solid-dose and parenteral manufacturing equipment, and slow adoption of continuous manufacturing processes.


A US government report on advanced manufacturing promotes continuous manufacturing of pharmaceuticals, which has had recent commercial success but faces challenges for widespread adoption.
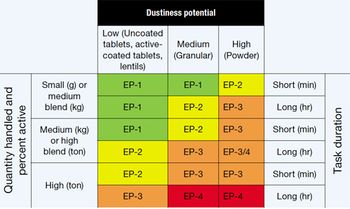
Safe handling of HPAPIs requires determining exposure potential and selecting appro-priate containment strategies.

FDA approved an update in the manufacturing of Prezista (darunavir) using a continuous manufacturing line at Janssen Supply Chain’s facility in Puerto Rico.

Texture analyzers can be used to evaluate wall hardness and elasticity of softgel capsules.

Pharmaceutical Technology spoke with Bill Randolph, vice-president, Technical Services, Janssen Supply Chain, about some of the considerations for technology transfer of a continuous, solid-dosage manufacturing process and what he sees as the outlook for continuous manufacturing.

Pharmaceutical Technology spoke with FDA to get the agency’s insights on how the industry can ensure quality in solid and semi-solid dosage products.