
Operator training, preventive maintenance, and regularly scheduled calibration help prevent the manufacture of off-weight tablets.

Operator training, preventive maintenance, and regularly scheduled calibration help prevent the manufacture of off-weight tablets.

Excipients for lowering formulation costs and improving lipid-based formulations and the use of melt-spray-congeal microsphere sachet technology for targeted controlled release attract attention at the 2013 American Association of Pharmaceutical Scientists Annual Meeting.
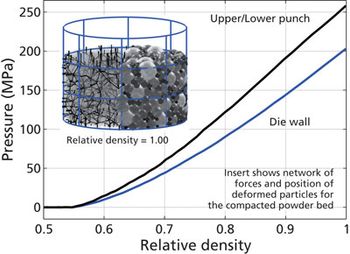
Screening methods and predictive models address tenacious tablet-sticking problems.

Pliva?s new Oral Solid Forms Facility will increase Pliva's production capacities for tablets and capsules.

Interview of Detlev Haack, head of R&D, Hermes Pharma on the characteristics, benefits and manufacturing Methods of effervescent tablets
HERMES PHARMA has pioneered user-friendly dosage forms. Our effervescent and chewable tablets, lozenges, orally disintegrating granules and instant drinks are convenient to use, easy to swallow and taste good.

An industry leader since 1933, Catalent develops and manufactures over 80% of the world's Rx softgel products with 200+ products on the market in 80+ countries.

The collaboration with Pfizer aims to develop next-generation, continuous manufacturing for solid-dosage pharmaceuticals.

Foam granulation is much easier to control under mechanical dispersion conditions, which is where most industrial processes operate.

The Bosspak VTC 100 electronic tablet and capsule counter from Romaco?s is designed to fill pharmaceutical solids or food supplements into bottles at high speed.

Direct tablet compression is simpler than wet or dry granulation, but obtaining the right flow properties to ensure good compaction and uniform drug distribution can be a challenge. Porous silica gel, when used as a glidant, can address these issues.

Experts, including researchers at I Holland, Natoli, and Rutgers University’s Engineering Research Center for Structured Organic Particulate Systems, are seeking a greater understanding of the fundamental causes of tablet sticking and are developing predictive models to more quickly find solutions to specific sticking problems.

Risk management guides decisions in facility design and operation for highly potent drugs.

Greater automation and the adoption of solid versus perforated pans are some recent advances.

Dynamic testing and advances in shear testing provide better insight into powder physical properties and external variables that affect powder behavior.

Crushing, fracturing, and bending tests quantify hardness.

Drug manufacturers today are increasingly challenged to find new, effective methods for the delivery of active pharmaceutical ingredients, and many are discovering that orally disintegrating technologies can help.

Podcast interview with Nick Johnson, Strategic Marketing Director for Modified Release Technologies at Catalent Pharma Solutions


The trick to taste-masking in solid dosage forms is to never let the taste buds have a chance.

Industry is moving toward closed-loop control of continuous processing.

Equipment suppliers are helping the pharmaceutical industry move towards adoption of continuous tablet production.

Loss-in-weight feeders provide high accuracy for feeding powders.

An alternative chapter has been added to the European Pharmacopoeia for dosage uniformity.

New product reviews for February 2013.