
Hermes Pharma has commercially implemented hot-melt coating (HMC) technology at its production facility.

Hermes Pharma has commercially implemented hot-melt coating (HMC) technology at its production facility.

Integrated pharmaceutical blister-packaging equipment systems strengthen serialization and brand protection capabilities.

Experts at the ISPE annual meeting describe best practices, including containment and production in classified spaces.

AstraZeneca’s solid-dose facility in Taizhou, China received ISPE’s FOYA 2015 Overall Winner award.

The FDA grants to the Rutgers-led C-SOPS research consortium will support the introduction of continuous manufacturing techniques for pharmaceuticals.

The Krämer KCP10 upward-conveying capsule polisher elevates, polishes, and dedusts capsules.

Elizabeth Scheu & Kniss will supply replacement parts for Bosch capsule filling machines.

WuXi PharmaTech and TruTag Technologies successfully applied and detected TruTag's edible microtags on solid-dosage drug products and found no effect on dissolution.

A study of root cause in stability samples suggests the need for tighter control of the sodium lauryl sulfate manufacturing processes.

Sepha's VisionScan Max can leak-test full production batches of blister packs.

Vacuum-conveying conditions for powders and other free-flowing solid forms can be simulated in Volkmann's new laboratory.

ACG Worldwide discusses current and future uses of capsules in a video interview with Pharmaceutical Technology.
PAT holds the key to real-time quality assurance and consistent product quality in pharmaceutical manufacturing.

Appropriate feeding equipment can be selected to meet the challenges of dosing powders that are sticky or do not flow well.

Economic benefits, equipment availability, research results, and FDA support are driving progress in continuous processing.

Innovations for tablet tooling and presses improve quality and productivity.
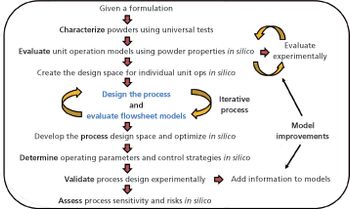
In-silico design facilitates process optimization and evaluation of process control strategies.

A drug-product manufacturing classification system (MCS) for oral solid-dosage forms is proposed by an Academy of Pharmaceutical Sciences working group.

Penn Pharma's equipment addition meets growing need for continuous dry granulation and for handling of highly potent drugs.

Innovative equipment, analytical techniques, software, and modeling systems are improving the tableting process.

Analytical technologies must accurately identify and measure the critical material attributes of APIs and excipients.

Ionic liquid technologies offer a new way to improve bioavailability.

New cellulosic polymers have been shown to improve solubility in these key amorphous solid dispersion processes.

MG America's TEKNA Capsule Filler has advanced controls to reduce powder loss and maintain optimal performance.