
More efforts are needed to raise awareness of biosimilars among physicians and patients in Europe and address scepticisms about the quality and safety of biosimilars.

More efforts are needed to raise awareness of biosimilars among physicians and patients in Europe and address scepticisms about the quality and safety of biosimilars.

The Thermo Scientific TruScan RM Analyzer features a chemometrics package that allows users to build customized models for both qualitative and quantitative sample predictions.

The agency publishes draft guidance on assessing the adhesion of transdermal delivery systems and topical patches.

Xellia, a generic anti-infective drug manufacturing company, is constructing a laboratory services building at its manufacturing site in Budapest, Hungary.

W.R. Grace & Co. will sell its chromatography product lines, which includes chromatography instruments, columns, and other related products.

Packaging technologies for serialization, parenteral products, and solid-dosage forms were displayed at INTERPHEX 2016.

A US government report on advanced manufacturing promotes continuous manufacturing of pharmaceuticals, which has had recent commercial success but faces challenges for widespread adoption.


In this article, the authors look at the limitations of the validation for a single-use shipping system and provide a perspective on what shipping validation means.

This article examines the changes now taking place, and what the future might bring.

Collaboration and single-use technologies aided the rapid scale-up of Ebola vaccine manufacturing

Intact enables aseptic filling in non-classified environments, maintains sterility throughout the process, and reduces costs, timelines, and contamination risks.

This article discusses the issue and offers food for thought and suggestions for more science-driven approaches.

*Updated May 11, 2016Following JHL Biotech’s opening of a FlexFactory flexible manufacturing facility in Hsinchu, Taiwan in 2014, the company opened a second manufacturing location in Wuhan, China on May 10, 2016. According to the company, with the addition of the new Wuhan facility, JHL Biotech will now hold the largest volume of single-use cell-culture capacity in all of Asia.

The agency cited Apotheca Supply, a repackager and relabeler of pharmaceuticals for CGMP violations.

The Cell and Gene Therapy Catapult released a review of their third annual survey of GMP cell and gene therapy facilities in the United Kingdom.

Under the terms of an agreement with Pfizer, WAVE will advance up to five programs from discovery through the selection of clinical candidates.

Progress is being made, but work remains to be done. Brian Daleiden, vice president of marketing at TraceLink, discusses the state of the industry, as companies attempt to meet complex, and changing, global requirements.

MilliporeSigma expands its Carlsbad, California-based GMP capacity for viral and gene-therapy products by nearly 90%.
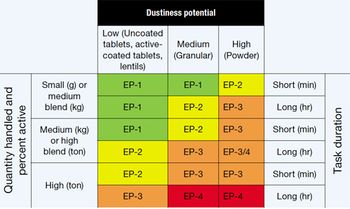
Safe handling of HPAPIs requires determining exposure potential and selecting appro-priate containment strategies.

Experts discuss some of the emerging trends in bioprocessing in 2016, including 4D bioprinting, 2D-NMR, and the CAR-T design space.

Bioprocess operations-from cell line selection to final filtration-can influence the consistency and purity of biologic drug substances.

Troubleshooting and collaboration are essential in implementing commercial lyophilization processes.

With deadlines only a few years away, some companies have not started serialization programs, while others are taking a tactical, short-term approach, losing out on potential business benefits.

Armin Gerhardt, associate professor of Pharmaceutical Science, Concordia University Wisconsin School of Pharmacy, discusses the effects of moisture on product quality and how to achieve good control of moisture during pharmaceutical manufacturing operations.