
National Bulk Equipment’s material handling system for non-free flowing materials can be specified to meet cGMP cleanability standards.

National Bulk Equipment’s material handling system for non-free flowing materials can be specified to meet cGMP cleanability standards.

Pharmaceutical Technology spoke with Franco Negron, president of Drug Product Services at Patheonl, about sustainable technology and reducing energy costs.

Curida entered into a partnership with René Bommer, PhD, founder and owner of pharmAccel Consulting.

Q&A with David A. Steil, pharmaceutical market manager, Camfil Air Pollution ControlPharmTech: Recently there has been an emphasis on green and sustainable technology in the pharmaceutical/biopharmaceutical industries. In what ways does Camfil APC’s technology contribute to sustainability?

Manufacturing of antibody drug conjugates requires high-containment solutions, such as high-performance aseptic isolators.

Regular removal of residues from disinfectants and sporicidals is important for improved aesthetics and safety in cleanrooms.

The course, intended for healthcare professionals, provides an overview of biosimilar products and FDA’s biosimilar product development programs.

Smart glasses enhance remote troubleshooting and process management for pharmaceutical manufacturing.

Near-field communication labels communicate with consumers and support personalized medicine.

Smart glasses enhance remote troubleshooting and process management for pharmaceutical manufacturing.

Biocorp’s Easylog smart sensor for insulin delivery devices enhances compliance.

Innovations awarded at Pharmapack Europe include improvements for patient compliance and protection from errors, tampering, or misuse.

There are no clinically meaningful differences between Celltrion’s CT-P13 and Remicade, according to an FDA briefing released ahead of the formal panel meeting.

The agency prepares a plan to implement new packaging safety features.

When implementing disposable technology for aseptic processing, considerations include material compatibility, material sourcing, facility layout, and training.

AMETEK announced the acquisition of Brookfield Engineering Laboratories, a Massachusetts based manufacturer of viscometers and rheometers.

LabConnect built a biorepository facility in Tennessee.
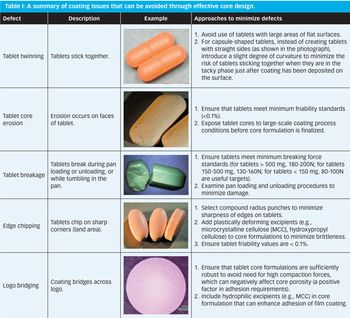
Common defects in tablet film coating can be minimized by effective design of the tablet core and the coating process.

Scale-down modeling is instrumental in supporting the development of downstream biopharma manufacturing processes.

The International Society for Pharmaceutical Engineering (ISPE) Facility of the Year Awards (FOYA) program announced its 2016 Category Award winners for operational excellence, sustainability, process innovation, project execution, equipment innovation, and facility integration.

Hazardous reagents can simplify processes and provide higher yields and purities.

Catalent plans a $4.6 million investment to expand secondary packaging and storage in Asia.

Sustainable harvesting combined with CMO expertise helped Centroflora CMS ensure supply continuity after it acquired Boehringer Ingelheim's non-captive API phytochemicals portfolio.

The Biosimilars Forum launched Partnership for Biosimilars Education and Access, an education initiative raising awareness of biosimilars in the US.

Mergers and acquisitions have changed the shape of the contract services market as big players seek to build full-service capabilities.