
Grand River Aseptic Manufacturing expands disposable technology capabilities at aseptic facility.

Grand River Aseptic Manufacturing expands disposable technology capabilities at aseptic facility.

AstraZeneca partners with the Wallenberg Center for Protein Research to conduct studies on the Secretome.

PSL has installed several advanced contained filtration and drying facilities as part of an expansion of a pharmaceutical manufacturing plant in Singapore.

Ajinomoto Althea expands biological drug product manufacturing operations to include ADCs.

Sandoz announces that the European Medicines Agency has accepted a MAA for a biosimilar of etanercept.

Catalent has entered into an exclusive long-term supply agreement to produce Pfizer’s OTC proton-pump inhibitor for heartburn treatment, Nexium 24HR (esomeprazole), which is also marketed as Nexium Control outside the US.

FDA released an interim response to AbbVie’s citizen petition on the labeling of biosimilars.

TxCell signs strategic agreement with MaSTherCell for European manufacturing of its cell therapy products.

FDA sets a July 2016 deadline for the final version of the rule on labeling changes for approved drugs and biologics.

Paragon Bioservices entered into a contract with the International Aids Vaccine Initiative for the process and analytical development and cGMP manufacturing of an HIV vaccine candidate.

Biogen, Genentech, Johnson & Johnson, Novartis, and Patheon publicize their support for action on climate change.

Seqirus, CSL Limited’s influenza vaccine business, announced the opening of their corporate headquarters in the United Kingdom.

Microorganism lethality requirements for process validation must always be balanced with the need to protect product integrity and patient safety.

All openings and potential apertures for air penetration must be considered when designing a cleanroom so that the HVAC system can maintain the desired negative or positive pressure.

FDA confirmed quality focus while Congress moved to bolster biomedical innovation.

The plant will manufacture Clariant’s moisture-control products to support the growing pharmaceutical packaging market in India.Clariant announced on Dec. 1, 2015 that it is investing CHF 10 million in a new packaging manufacturing plant in Cuddalore, India. The plant will manufacture Clariant’s moisture-control products to support the growing pharmaceutical packaging market in India.

The author provides an overview of the key aspects of the current Canadian pharmaceutical market.
Quality by design, in-vitro release testing, and modern analytical methods are improving understanding and control of these complex formulations.

The results of an industry workgroup’s examination of EMA’s guide on shared facilities are presented.

Amgen announced the submission of a BLA with the FDA for ABP 501.

Xcelodose provides automated processing of API directly into capsules at low doses. The expansion of Xcelodose reinforces Juniper’s early stage capsule filling capability.CDMO Juniper Pharma Services, a subsidiary of Juniper Pharmaceuticals, announced that it has expanded its Xcelodose powder micro-dosing system. This move reinforces the company’s early stage capsule filling capability.
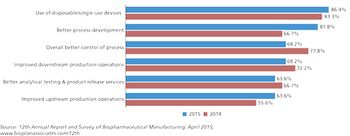
Better process development is creating industry benchmarks for bioprocessing.

A study backed by the Centre of Regenerative Medicine found that laminins are crucial cell components that could help aid the commercial production of stem cell therapies.

The Safe Chain Track and Trace System automates traceability for supply chain security.

CSafe partners with Lufthansa Cargo in dedicating a fleet of RKN containers to Cool/td-Active service offering.