
Hermes Pharma has commercially implemented hot-melt coating (HMC) technology at its production facility.

Hermes Pharma has commercially implemented hot-melt coating (HMC) technology at its production facility.

NICE announces plans to back biosimilar alternatives to Merck’s Remicade.

Baxter has voluntarily recalled lots of IV solutions due to potential container leakages and particulate matter.

Pharma companies reduce the impact of manufacturing on the environment with innovations in energy and waste treatment.

The European Pharmacopoeia rewrites in its general chapter on Raman spectroscopy.

The Alfa Laval Safety Valve, a true spring-loaded safety valve, is designed to protect both equipment and people.

FuelCell Energy plans to install a 5.6 megawatt fuel cell power generation system for Pfizer to provide reliable and low carbon electricity and steam for its 160-acre R&D facility in Groton, CT.

The company has voluntarily recalled one lot of magnesium sulfate in water for injection because of incorrect labeling.

Takeda Pharmaceuticals announced the acquisition of a biopharmaceuticals manufacturing plant in Minnesota.

The Cell Therapy Catapult, University of Birmingham, and Cancer Research Technology collaborate on CAR-T cell immuno-oncology therapy development.

FDA discusses a new program that allows pharmaceutical companies to submit proposals for new manufacturing technology.

Jones Packaging and ThinFilm will collaborate on NFC OpenSense technology for pharmaceutical packaging.
Parenteral packaging of the future will include more automated lines, ready-to-fill packaging formats, and supply-chain transparency.

DAVID LEAHY/GETTY IMAGESThe pharmaceutical industry has an important role to play in implementing solutions to global envi
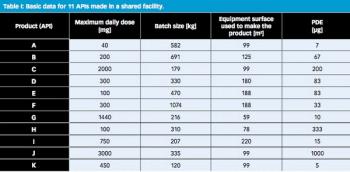
The 10-ppm criterion for the acceptable concentration of potential API in cleaning validation to minimize cross contamination into next product has been employed for many years. This article describes why the 10-ppm criterion, which was established based on analytical limitations and estimates of acceptability, is no longer necessary and why a risk-based approach should be universally adopted.

The revised USP Chapter 1207 gives best practices for obtaining reliable data in container closure integrity testing.

Amid corporate restructurings, regulatory initiatives, and aging R&D assets, will drug development accelerate or stall in 2016?

The Ross AMK Kneader Extruder combines the technologies of a double-arm mixer with an extrusion screw.

The BioClamp Plastic Tri-Clamp, from BioPure Technology, part of the Watson-Marlow Fluid Technology Group, is a patented plastic union tri-clamp meant to reduce distortion on polymeric fittings when subjected to heat.

Mass Spec Lab, a privately owned analytical company, announced its official launch on Dec. 28, 2015 in Southern California.

Boehringer Ingelheim announced it will establish a new biopharmaceutical production facility in Vienna.

Samsung BioLogics begins construction on their third facility in Songdo, Korea.

GENEWIZ signs definitive agreement to acquire Beckman Coulter's gene services business.

Recipharm has signed an agreement with Sweddish pharmaceutical company LIDDS for the production scale up and manufacture of LIDDS’ Liproca depot for the treatment of prostate cancer.

The agency has launched a new web platform to foster scientific innovation.