
Novo Nordisk broke ground on a facility in Clayton, NC, to manufacture APIs for GLP-1 and insulin medicines.

Novo Nordisk broke ground on a facility in Clayton, NC, to manufacture APIs for GLP-1 and insulin medicines.

The Matrix Alliance was formed by Vanrx Pharmasystems with inaugural members ARaymond Life, Daikyo Seiko, Datwyler Group, Ompi, Schott AG, Schott Kaisha and SiO2 Medical Products.

Collaboration will provide for unified development and manufacture of antibody drug conjugates.

The company broke ground on a $79 million production facility at its Ravensburg Schuetzenstrasse site.

The agency announced enhanced warnings for immediate-release opioids to inform prescribers and patients of risks related to use.

The agency cited Emcure Pharmaceuticals with CGMP violations.

Thermo Scientific TruTools chemometrics package extends the capabilities of the portable TruScan RM Raman Analyzer.

Advanced electronic batch recording (EBR) manages workflows and recordkeeping for compliance and production efficiency.

Revised versions of ISO 14644 adopt changes to sampling procedures and monitoring plans for cleanrooms.

The author discusses key areas of focus and presents best practices from the International Pharmaceutical Excipients Council (IPEC).

The author discusses issues and best approaches for solid dosage form pharmaceuticals.

This article explores two commercial platforms, and touches on a new program underway in the UK.

Matt Richardson of Capsugel and Michael Morgen of Bend Research, discuss improvements and results that have been seen in the second generation of hydroxypropyl methyl cellulose (HPMC) materials.

Wear-resistant materials and coatings protect tablet punches and dies from abrasive formulations.

The syriQ Rigid Cap (SRC) and SCHOTT TopPac Rigid Cap (TRC) closure systems from Schott use an intuitive twist-off mechanism for ease of use and container closure integrity.

Hovione will operate a commercial-scale continuous manufacturing facility in New Jersey as part of an agreement with Vertex Pharmaceuticals.

The module was installed in the company’s Watson laboratory information management system in Poitiers, France.
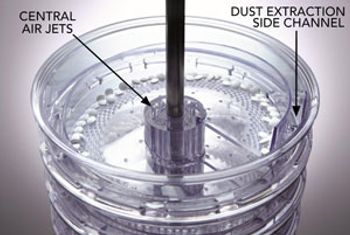
Dedusting equipment and techniques address problems associated with tablet manufacturing dust accumulation.

The inspection confirmed that the facility was compliant with GMP guidelines.

Integrated data and cloud-based solutions can be used for process optimization.
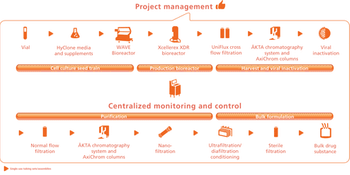
Asking the right questions is crucial to establishing a biopharmaceutical facility design.

Scale-down modeling accelerates the development of downstream biopharma manufacturing processes.

Analytical laboratory equipment adapts for new realities of downsizing, outsourcing, and speed demands.

Choosing the right facility size requires tailoring the design for current needs as well as anticipating the future.

TxCell’s new facilities are based in Sophia Antipolis, France, on the premises of GenBiotech.