
The European Medicines Agency launched on December 20, 2010, a public registry about small and medium-sized enterprises (SME) that includes information about SME-registered companies.

The European Medicines Agency launched on December 20, 2010, a public registry about small and medium-sized enterprises (SME) that includes information about SME-registered companies.

Novartis (Basel) signed a memorandum of understanding with the City of St. Petersburg, Russia, confirming its intent to build a new full-scale pharmaceutical manufacturing plant in St. Petersburg.

Pfizer Recalls One Lipitor Lot; Sanofi Aventis Names Head of R&D; and More.

A US Food and Drug Administration inspection completed on Dec. 9, 2010 revealed persistent deficiencies at McNeil Consumer Healthcare's Fort Washington, Pennsylvania, facility.

A concentrated course providing the attendee an opportunity to get and keep right up to date with essential standards and best practice for cleanrooms intended for life sciences applications.

Thermo Fisher to Acquire Dionex; Former SOCMA CEO Joe Acker Dies; and More.

Abbott Laboratories (Abbott Park, IL), B. Braun Medical (Bethlehem, PA), and Roxane Laboratories, a subsidiary of Boehringer Ingelheim (Ingelheim am Rhein, Germany), agreed to pay the United States $421 million to settle False Claims Act allegations.

Equipment and Processing Report talked to Jonathan Seville, dean of the School of Engineering at the University of Warwick, to find out how positron-emission particle tracking could help pharmaceutical manufacturers gain process understanding.

Pfizer (New York) appointed Ian C. Read, currently head of the company's global biopharmaceutical operations, as president, chief executive officer, and director. He succeeds Jeffrey B. Kindler, who retired from the company.

Company and People Notes: GlaxoSmithKline acquires Nanjing MeiRui Pharma; Cephalon's CEO to remain on medical leave; and more.

Editors' picks of pharmaceutical science and technology innovations.

Supportive public policy is needed in order for innovation to flourish.

Outsourcing packaging functions can save time and money.

World AIDS Day 2010 reminds us that prevention and hope can help fight the disease.

Sometimes doing what you think is right ends up being completely and utterly wrong.

Covance's deal with sanofi-aventis demonstrates the power of scale and scope.

The author explains the idea of equivalence and describes how it can facilitate equipment validation.
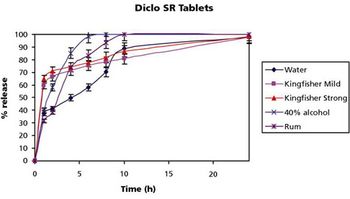
The authors evaluated the effect of alcoholic beverages on the release profiles of sustained-release dosage forms containing metformin and diclofenac.

GlaxoSmithKline (GKS, London) outlined a plan to invest EUR 500 million ($649 million) in research and development (R&D) and related manufacturing in the United Kingdom conditioned on the successful implementation of a so-called "patent box" measure by the UK government.

The growing uptake of single-use sterile packaging in pharmaceutical production processes mirrors the broader trend towards single-use across every sector of the pharmaceutical industry.

One of the biggest challenges in designing cleanroom doors for the pharmaceutical industry is in creating a sturdy door with a high hygienic performance that is light and easy to clean.

Modular cleanrooms are a flexible and adaptable solution that come on the back of a lorry and offer numerous benefits.

When it comes to monitoring the cleanroom, too much emphasis is often directed at the annual check, but ongoing monitoring is also crucial.

Contract Manufacturing Organisations offering manufacturing capabilities to suit specific industry sectors, niche segments or perhaps simply to meet the demands of individual clients? processes face a greater challenge when designing their facilities and cleanrooms when compared with an originator company or a single-product manufacturing facility.

With the increasing need for businesses to reduce costs and demonstrate value, there is a requirement to look at all aspects of bio/pharma drug development and manufacturing to achieve efficiency improvements.