
Brain and heart broth is still used in path labs, but, when it comes to biopharmaceutical manufacturing, cells are having to learn to do without animal components whenever possible.
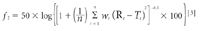

Brain and heart broth is still used in path labs, but, when it comes to biopharmaceutical manufacturing, cells are having to learn to do without animal components whenever possible.
Raman spectroscopy has become a commonly used technique for physicochemical analysis that possesses many advantages over other analytical techniques. It is a very attractive characterization tool, not least because it enables measurements in water. However, very few examples of its application in an aqueous environment exist in literature. This paper provides some recent applications of Raman spectroscopy in pharmaceutical material and process characterization when water is present.

Tokyo (Jan. 18)-Tanabe Seiyaku Co. Ltd. confirmed it has had discussions with Mitsubishi Pharma Corporation over a possible merger between the two companies, but that no definite decision has been made for such a deal.

London (Feb. 1)-AstraZeneca PLC unveiled a plan to improve asset utilization within its global supply chain that involves rationalizing production assets and cutting staff. The company made the announcement as part of its fourth quarter and full-year 2007 financial results.

Pfizer's restructuring plan provides yet another example of new supply-chain strategies by the pharmaceutical majors, which involve rationalization of manufacturing facilities and cost improvement. A review of these moves, an outlook for the pharmaceutical market in 2007, and analysis of US pharmaceutical production and trade.

Cardinal Health (www.cardinal.com) agreed to sell its contract services unit, Pharmaceutical Technologies and Services (PTS), to The Blackstone Group (New York, NY, www.blackstone.com) for roughly $3.3 billion in cash.

Dutch biotech company, Crucell N.V and technology partner DSM Biologics have signed a PER.C6 research licence agreement with AbGenomics Corporation based in Taiwan.

The US Department of Health and Human Services (HHS, Washington, DC) has awarded three vaccine makers a total of $132.5 million to advance their strategies for adjuvant-containing vaccines to combat the H5N1 strain of avian influenza. Under the contracts, each company will build capacity to produce either 150 million does of the vaccine or enough adjuvant for 150 million doses within six months after the onset of an influenza pandemic.

Biological firm, Scigen Ltd, has invested $30 million in a vaccine manufacturing facility in Israel.

In an effort to improve its revenues in the short and long-term, Pfizer, Inc. announced plans to reduce its workforce by 10%, close three manufacturing facilities, rationalize research and development operations, and restructure its US pharmaceutical operations. The announcement comes as the company expects its 2007 and 2008 revenues to be comparable to 2006 levels.

Indianapolis, IN (Jan. 11)-Eli Lilly and Company announced several strategic changes to its global manufacturing operations. The changes include termination of construction of a planned insulin manufacturing plant in Virginia, staff reductions in its operations for small-molecule active pharmaceutical ingredients (APIs), and investments in manufacturing biotech-based drug products

Brussels, Belgium (Jan. 11)-EPCglobal, the not-for-profit organization dedicated to driving global adoption of the Electronic Product Code (EPC), has ratified the electronic pedigree document specification.

Washington, DC (Dec. 28)-The US Department of Homeland Security (DHS) has proposed regulations for improving security at high-risk chemical facilities, a category that may include some pharmaceutical production facilities.

Darmstadt Germany (Jan. 8)-Merck KGaA closed on its roughly CHF 16.6 billion ($13.3 billion) deal to acquire a majority stake in the European biotechnology company Serono (Geneva, Switzerland), officially launched Merck Serono SA as a new entity within Merck KGaA, outlined its integration strategy, and announced plans to divest its generics business.

Berlin and Leverkusen, Germany (Dec. 29)? Bayer Schering Pharma AG was officially launched as a new company following the acquisition of Schering AG (Berlin, Germany) by the Bayer Group (Leverkusen, Germany).

Stockholm, Sweden (Dec. 15)-As part of a plan to expand its biotechnology production capacity, Pfizer, Inc. commissioned the construction company Skanska AB to build a plant in Strangnas, Sweden for manufacturing Pfizer's human growth hormone product "Genotropin" (somatropin [rDNA origin] for injection).

Hafnarfjordur, Iceland (Dec. 21)-The generic drug manufacturer Actavis Group is expanding its manufacturing capabilities in India and plans to close a facility in Norway.
Pore-size ratings are so unrelated to actual dimensions and so subject to anomalous interpretations as to make substantial dependency upon their values an unwise choice. Moreover, the means of measuring them are questionable. The pore-size rating system at best provides a qualitative differentiation.

Democrats are back on top in Congress and are mapping a broad agenda for change. Prescription drug pricing, medical product safety, and access to needed treatments are high on the priority list. Manufacturers will be in the hot seat answering questions about patent practices, high-risk products, and why drugs cost less in other countries than in the United States. The real challenge, however, will be to gain approval of a bill to reauthorize the Prescription Drug User Fee Act (PDUFA) before the program expires on Sept. 30, 2007. Such legislation also would renew user fees for medical devices and continue the pediatric drug exclusivity program.

Industry will be challenged to embrace new methods of supply chain collaboration.

There are currently several hundred biotech-based drugs in clinical trials, representing around a quarter of all drugs in development - a proportion that looks set to increase.
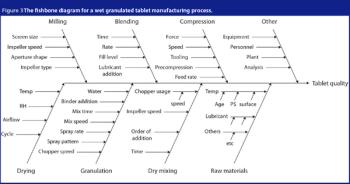
In recent years, AI has become important in a number of fields in helping to make better use of information, increasing efficiency and enhancing productivity.

Dec. 28, 2006 Company Notes: Albemarle, Clinical Data, Crucell, Merck, PDA, QLT, Sanofi Pasteur, Shimadzu

Pharmaceuticals current good manufacturing practice (CGMP) violations accounted for just 36 of the 441 Warning Letters issued by the US Food and Drug Administration in 2006.

US District Court for the Eastern District of New York Judge Joanna Seybert's Dec. 11, 2006 Order granting a preliminary injunction in RxUSA Wholesale, et al, v. FDA, barring FDA from enforcing some drug pedigree rules.