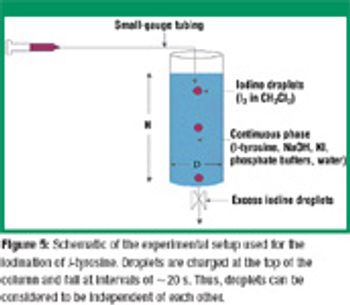
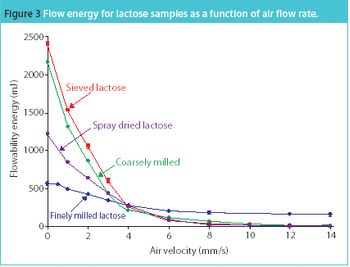
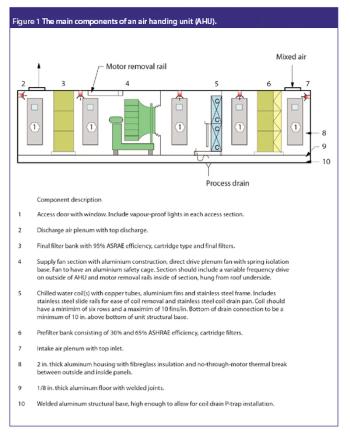
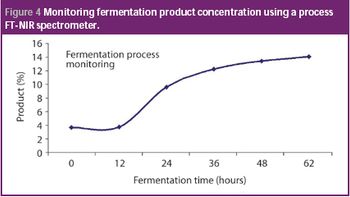
San Antonio, TX (Nov. 1)-The opening session of the ?Pharmaceutical Technologies? track at the 2006 Annual Meeting of the American Association of Pharmaceutical Scientists in San Antonio, Texas was standing room only as R. Christian Moreton, PhD, vice-president of pharmaceutical science at Idenix Pharmaceuticals, and Richard O. Mannion of Purdue Pharma discussed ?Product Development Using a Virtual Company Model.?