
The pharmaceutical industry plans moderate increases in spending for equipment and machinery in 2007 as expenditures hold steady.

The pharmaceutical industry plans moderate increases in spending for equipment and machinery in 2007 as expenditures hold steady.

A wealth of citations helps illustrate the applicability of the analytical methods and also highlights their pitfalls.

... there is a huge manufacturing challenge involved in bringing cell therapies (especially stem cell therapies) from the lab bench into the clinic.

So what is the EU to do? It must grant its younger researchers greater scientific autonomy and academic independence.

Missed or late calibration dates can accumulate, and even if the equipment is labelled appropriately, it can suggest poor management of resources and priorities.
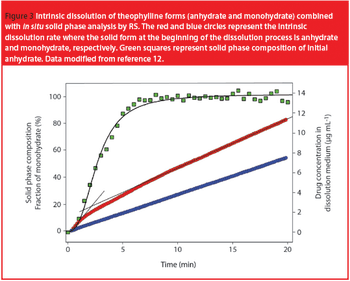
When a dosage form or an API is introduced to the gastrointestinal tract, or dissolution media mimicking it, a transformation from a metastable to a more stable form with lower solubility and bioavailability is possible.

Agawam, MA (Mar. 26)-Contract manufacturing and testing laboratory Microtest signed a manufacturing deal with Antisoma to produce AS1411, a new drug being developed for the treatment of various cancers.

South San Francisco, CA (Mar. 28)-Genentech, Inc. announced plans to invest $140 million in 1000-liter manufacturing facility in Singapore.

Midland, MI (Mar. 21)-The Dow Chemical Company formed an alliance with Colorcon, Inc. for the global marketing, sales, technical service and development, and distribution of Dow pharmaceutical excipient products for use in controlled-release applications.
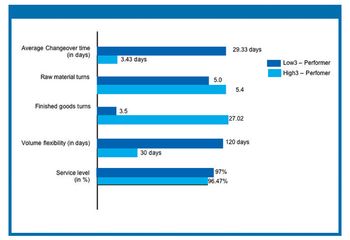
Improving performance at an active pharmaceutical ingredient manufacturing plant involves an integrated approach that incorporates methods for optimizing total production management, quality control and assurance, and inventory management. The authors analyze results from a recent benchmarking study to evaluate the critical success factors in high-performing API manufacturing plants.


Berlin and Basel, Switzerland (Mar. 26)-Bayer Schering Pharma AG and Novartis reached an agreement over multiple sclerosis therapy ?Betaseron.?


Paris (Mar. 15)-Sanofi-Aventis plans to close its manufacturing site in Waterford, Ireland.

Bangalore, India, (March 14)-Bristol-Myers Squibb Company is expanding its research and development (R&D) capabilities in India.

Kenilworth, NJ (Mar. 12)-Schering-Plough Corporation agreed to acquire Organon BioSciences N.V., the human and animal health businesses of Akzo Nobel N.V., for EUR 11 billion ($14.4 billion) in cash.

Gurgaon, Haryana, India (Mar. 13)-Ranbaxy Laboratories Limited confirmed that it has made a nonbinding bid for the generic drug business of Merck KGaA.

Bayer has confirmed plans to cut 6100 jobs globally.

"Now is the moment for a big push" in improving the environment for the UK biotech industry argues Tony Blair, Prime Minister.

Berlin, Germany (Mar. 2)-Estimating it would save approximately EUR 700 million ($917.7 million), Bayer HealthCare will integrate the activities of its Pharma division with those of the former Schering AG, Germany.

Just because the wheels are turning doesn't mean they're going forward.

Security, the environment, aging populations, the bio-boom, and cost control are just a few of the drivers that will influence pharmaceutical packaging for the remainder of this decade.

FDA's new safety program, Critical Path Initiative, and user-fee proposal seek to reinvigorate pharmaceutical R&D.

Filling machines often are installed in sterile rooms and separated by isolators to prevent contamination. These methods have certain drawbacks, including making interventions more difficult. Restricted-access barrier systems are an alternative that ensures sterility and facilitates interventions.