
New York (Apr. 26)-Bristol-Myers Squibb Company and Pfizer, Inc. entered into a collaborative agreement for the development and commercialization of apixaban, an anticoagulant that BMS discovered.

New York (Apr. 26)-Bristol-Myers Squibb Company and Pfizer, Inc. entered into a collaborative agreement for the development and commercialization of apixaban, an anticoagulant that BMS discovered.

Interphex2007, New York, NY (Apr. 26)-Robustness studies for analytical methods are critical in being able to provide the assurance to the quality of an analytical method, a topic addressed in a conference session, "Performing Analytical Method Validation Robustness for Regulatory Compliance," at Interphex on Thursday.

Interphex2007, New York, NY (Apr. 26)-Maintaining reliable equipment in pharmaceutical manufacturing operations is critical not only for achieving productivity goals and safety, it also is a prerequiste for moving operations to a lean-manufacturing environment.

Interphex2007, New York, NY (Apr. 26)-As focus shifts away from "one-size-fits-all" medicines and begins centering on personalized medicines, many companies begin to wonder how they can manufacture such drugs economically. Mark Hirschel, chief scientific officer of BioVest International, addressed that issue in his presentation, "A Bioreactor System Designed for Production of Personalized Therapeutics," at Interphex on Thursday.

Interphex2007, New York, NY (Apr. 26)-Counterfeit medicines are of increasing concern to the pharmaceutical industry, both because of the potential health risks to patients and because of the effects on pharmaceutical companies' businesses. While technology such as e-pedigree and radio frequency identification (RFID) represent solutions to secure the supply chain, there are challenges.

Interphex2007, New York, NY (Apr. 25)-As manufacturers prepare for processing more-potent compounds, they seek effective clean-in-place (CIP) and wash-in-place (WIP) design systems.

Interphex2007, New York, NY (Apr. 25)-One of the challenges facing pharmaceutical manufacturers is how to implement process analytical technology (PAT) into their commercial manufacturing processes. Michael Abad, engineering section manager, Abbott Laboratories shared insight from a project within Abbott in his presentation "Engineering for PAT" at Interphex today.

Interphex2007, New York, NY (Apr. 25)-As governments begin to contemplate the possibility of biological terrorism or a pandemic event, a new problem begins to emerge: in the case of a pandemic or an attack, even if a vaccine or treatment exists, how could it be produced in sufficient numbers to prevent the deaths of millions of people? That question was addressed in at the conference session, "Responding to Bioterrorism and Pandemic Events: A Case for Development of Flexible Manufacturing Space for Vaccine Production," at Interphex today.

Interphex, New York, NY (Apr. 25)-Problems caused by the presence and movement of air in pharmaceutical powders are increasing, and many times these problems can be avoided. James K. Prescott, senior consultant at Jenike & Johanson reviewed these problems in his presentation, "Interstitial Air Effect on Powder Flow," at the Wednesday conference session at Interphex.

Interphex, New York, NY (Apr. 24)-Compared with other industries such as the automotive industry, the pharmaceutical industry has been slow to adopt lean manufacturing. This situation, however, is changing.

Lyon, France (Apr. 17)-Sanofi Pasteur, the vaccine division of the Sanofi-Aventis Group, announced that the US Food and Drug Administration has licensed its H5N1 vaccine, making it the first avian-influenza vaccine for humans in the United States.

Billerica, MA (Apr. 13)-Millipore Corporation has become the first company to integrate radio frequency identification (RFID) technology with the filtration products used to manufacture biopharmaceutical drugs.

Toronto (Apr. 17)-Patheon, Inc. plans to restructure its current network of six pharmaceutical manufacturing facilities in southern Ontario, Canada as part of its strategy to focus on manufacturing prescription pharmaceutical products and to improve its profitability.

Chalfont St. Giles, UK (Apr. 16)-GE Healthcare, a unit of General Electric, acquired Wave Biotech LLC, a supplier of disposable manufacturing technologies and processing equipment for the biopharmaceutical industry.

Rockville, MD (Apr. 5)-The US Food and Drug Administration concluded its reinspection of Wyeth?s manufacturing facility in Guayama, Puerto Rico. Conditions at the Guayama facility prompted a Warning Letter in May 2006.

Washington, DC (Apr. 5)-The Pharmaceutical Research and Manufacturers of America joined the debate on follow-on biologics when senior vice-president Caroline Loew issued a statement recommending caution.

London (Mar. 29)-GlaxoSmithKline (GSK) plans to invest EUR 250 million ($334 million) at its production site at Currabinny, County Cork, Ireland over the next five years, according to a release issued by the Irish Development Agency (IDA Ireland, Dublin).

Washington, DC (Apr. 3)-The Synthetic Organic Chemical Manufacturers Association (SOCMA) adopted a policy on Inherently Safer Technology (IST) at its March meeting.

Singapore (Mar. 29)-The contract manufacturing organization Lonza Group (Basel, Switzerland) and Bio*One Capital (Singapore) broke ground for their large-scale commercial mammalian cell-culture manufacturing facility at Tuas Biomedical Park in Singapore.

Thousand Oaks, CA (Apr. 3)-Amgen, Inc. has provided a new schedule for the completion of a new manufacturing facility in County Cork, Ireland.

Washington, DC (Apr. 2)-The US Department of Homeland Security released an interim final rule that imposes federal security regulations for high-risk chemical facilities.
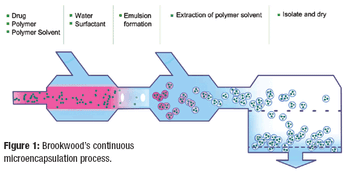
Alternatives to batch processing finally are starting to be taken seriously by pharmaceutical manufacturers, but the implemention of continuous processing is still in its infancy, and many challenges remain.

Many radio frequency identification projects are moving beyond the pilot stage, supported by new hardware and software tools.
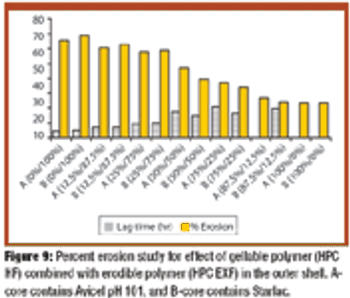
The authors prepared and tested press-coated tablets with various weight ratios of ethylcellulose to hydroxypropylcellulose (HPC) and various ratios of two different batches of HPC as an outer coating shell and fillers in core tablets. The tablets were examined for changes in time lag and release patterns of salbutamol sulfate.
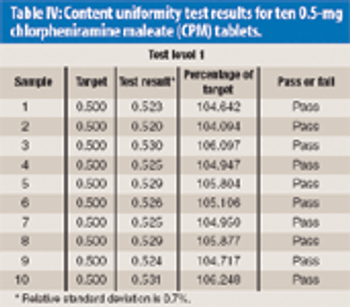
Near-infrared (NIR) assay and content uniformity of tablets provide fast, accurate means of monitoring tablet production that are in step with FDA's process analytical technology initiative.The authors discuss the process for testing a newly released NIR tablet analyzer to determine instrument precision and accuracy using chlorpheniramine maleate tablets.The data show promising results that could relieve laboratory workload of high-performance liquid chromatography analysis and bring analysis closer to real time for process monitoring.