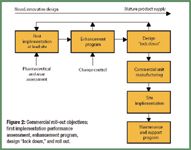
"Quality by design" (QbD) and "quality risk management" at long last seem to be moving from the buzzword stage to becoming important influences on drug development and manufacturing. A series of quality standards issued by the International Conference on Harmonization (ICH) is encouraging the adoption of common quality-based drug manufacturing approaches designed to reach the "desired state" of drug manufacturing (i.e., more efficient, agile, flexible operations that can reliably produce high-quality drug products with less regulatory oversight). These developments reflect increased pressure to make pharmaceutical manufacturing more efficient and less wasteful and to encourage regulators in all regions to focus on the most critical issues affecting product quality and patient safety.