
The American society of Mechanical Engineers's Council on Codes and Standards has formed 10 subcommittees to update and broaden the scope of the BPE standards.

The American society of Mechanical Engineers's Council on Codes and Standards has formed 10 subcommittees to update and broaden the scope of the BPE standards.

Comprehensive Web sites, journals, and other sources help pharmaceutical makers find packaging-related data quickly and easily.

Technologies ranging from new expression systems to PAT-inspired analyzers are driving biotech manufacturing forward to keep up with demand.

pharmaceutical science and technology innovations

Pharmaceutical Science & Technology Innovations

pharmaceutical science and technology news

Added functionality excipients facilitate the development of novel drug delivery methods and improve processing techniques.

Currently, high-production rates and continuous production processes favor existing tableting technologies. However, if tablet development becomes rate-limiting in the future, alternative technologies may prove attractive.

Some of the common problems faced by formulators and how using ion exchange resins may be able to solve them are discussed.
The article describes the basic principles of blow-fill-seal (BFS) technology together with the advantages it offers. Although BFS technology is an ideal process for aseptic filling of liquid pharmaceutical products there is still a risk of contaminating the product inside the filling area. This, together with regulatory requirements for the microbiological control of critical areas in pharmaceutical production, makes microbiological monitoring a necessity.

Recent technology improvements have made acrylics the preferred system for the aqueous enteric coating of tablets.

The content and quality of information supplied with drug products are among the most specifically defined areas associated with the products for sale. For patient or drug user, the information is presented in patient information leaflets (PILs) placed in the package. The readability testing of PILs is an important stage in the process of making the texts contained in the summary of product characteristics comprehensible to users, as this article discusses.
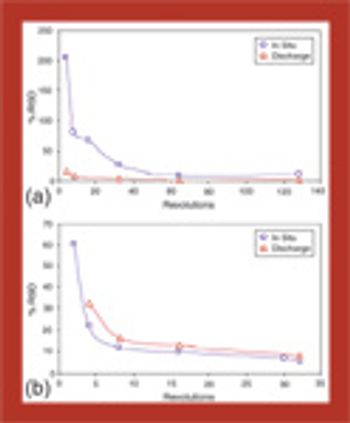
In this series of articles, bin blender performance is comprehensively reviewed using both free-flowing and cohesive mixtures. In part 1, an introduction to tools and techniques is presented, followed by an examination of parameter effects, mixing mechanisms, and the effects of cohesion on mixing.

The author describes a separation method for two active ingredients in the contraceptive pill with liquid chromatography UV detection.

Pharmaceutical Science & Technology Innovations

New technologies, along with changes in FDA oversight, may provide a critical path to make more new drugs available more quickly to patients.

Drug makers and their suppliers will need good planning and organization to meet the new bar coding requirements for drug products used in hospitals.

From data acquisition to enterprise resource planning, software systems operating at all levels of pharmaceutical manufacturing prepare for the seemingly inevitable implementation of process anlytical technology.

Pharmaceutical science and technology innovations

Bin blender performance is comprehensively reviewed using both free-flowing and cohesive mixtures.

Pharmaceutical Science & Technology News

Total organic carbon (TOC) analysis is a fast and effective analytical technique for cleaning validation. Understanding the various types of TOC technologies is essential for choosing the best solution.
This article describes the formulation of a tablet for a specific purpose, primarily using fractional or full factorial designs. The formulation work generated a matrix that was processed by two software packages based on neural networks. When the dataset was divided into smaller subsets, the agreement between the predicted and observed tablet properties of the optimized formulations was reasonable.

FDA's draft guidance on aseptic processing contains some inherent difficulties, including unrealistic expectations of sterility and microbial quantification, an absence of harmonization with international rules, and failure to support new technologies or a risk-based approach. The authors propose a science-based alternative.
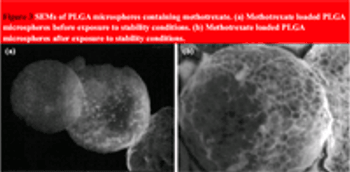
In this article, the authors describe a study into the factorial effect of selected process parameters on the pharmaceutical characteristics of poly(DL-lactide-co-glycolide) microspheres containing methotrexate. A study of the microspheres' stability at refrigerated temperatures is also examined.