
FDA is re-engineering the CMC review process for innovator and generic drugs and backing risk-based ICH quality standards.

FDA is re-engineering the CMC review process for innovator and generic drugs and backing risk-based ICH quality standards.

Emerging high-tech solutions prepare to cater to industrial automation with promises of increased efficiencies, faster communications, and custom applications.

Pharmaceutical Science & Technology News

The authors suggest a design strategy for an aseptic process simulation that focuses on the basic repeating unit of the process, establishing alert and action criteria for the unit itself, and using worst-case simulations to establish routine operational parameters for the manufacturing process.

A straightforward approach reveals how the probability of passing the USP content uniformity test can be calculated for tablets and capsules.

In this final part of a series of three articles, the results from experiments involving cohesive materials are discussed in terms of mixing performance, agglomerate comminution, and lubrication of powder mixtures.

Particulate generation and durability concerns are encouraging pharmaceutical manufacturers to seek alternatives to wood pallets.

The author describes the efforts of the standards committees to establish guidelines for PAT implementation and the impact of the guidelines on the industry.

A novel probe design can reduce the powder-sample density variance such that moisture and solvent levels can be accurately modeled and predicted in process.

FDA recently approved the first PAT applications for the introduction of rapid microbial testing of drug products and pharmaceutical-grade waters. Officials from FDA and GlaxoSmithKline worked together to ensure the appropriate scientific evaluation of the methods. Team members report on the successful validation approach and identify technical issues to be considered for the future.

The use of stable isotopic analyses to characterize drug products before they leave the manufacturer is explored as a means to identify counterfeit products.

Pharmaceutical Science & Technology News

Pharmaceutical science and technology innovations

The results of forced degradation studies indicate the need for alternatives to valerophenone as an internal standard calibration for quantifying ibuprofen in bulk drug and tablet assay samples.

Pharmaceutical Science & Technology Innovations

This month's column highlights some of the latest innovations in packaging seen at this year's Interphex conference.

The authors examine the VHP resistance of microbial isolates recovered from controlled environments and compared them with commercially available biological indicators under various test conditions.

Rapid structural elucidation of compounds in complex mixtures is a powerful technique in metabolite, degradation and process control applications. The ability to follow the fragmentation pathway through sequential MSn transitions provides added confirmation and increases the selectivity for monitoring compounds of interest in a complex mixture. Previously, the limiting factors in applying this technique were sensitivity at MS4 and higher transitions, and the cycle time required to acquire multiple MSn spectra across a narrow LC peak. Recent developments in trapping, detection efficiencies and scan rates have reduced these limitations and enabled rapid characterization of multiple compounds from single chromatographic runs.

Resolving the asset headache - the author explains how effective asset management can enable pharmaceutical companies to boost operational profit, increase production uptime and adhere to regulatory standards.
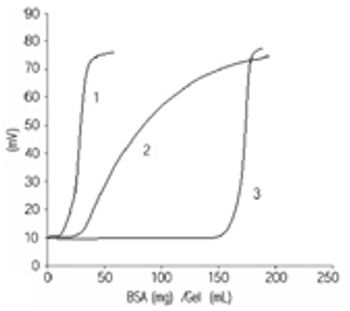
Tosoh has developed a new product - Toyopearl Toyoscreen that allows fast identification of the optimal resin for your sample. You can develop the optimal conditions for the separation in addition to the determination of the dynamic binding capacity. All the resins prepacked in these columns are real process resins concerning particle size. Results on the small columns can be scaled up to production.

If an inspection reveals any shortcomings ... a manufacturer may be warned, fined, or its facility closed down until full control can be demonstrated to the satisfaction of the authorities ....

Automated actuation of nasal sprays removes operator bias during measurement of particle size, spray-plume geometry, and delivered dose.

The electronic tongue technology provides a technically suitable and cost-effective method for screening and directing taste formulation, while eliminating both safety concerns and subjective bias.

Pharmaceutical Science & Technology News

In part two of a series of three articles, mixing rates and mechanisms are examined using rectangular bin blenders and two free-flowing mixtures.