
It Helps to be Flexible: Supple WIN Compounds Could Cure the Common Cold

It Helps to be Flexible: Supple WIN Compounds Could Cure the Common Cold

Cytogen and Dowpharm to Develop Targeted Anticancer Product


New Graduate Program Focuses On Pharmaceutical Technology

New Chemical Reaction Method Accelerates Indole Production

Bedford Recall

GSK and FDA Agree on Consent Decree

Purdue Pharmaceutical Manufacturing and Education Center Opens its Doors
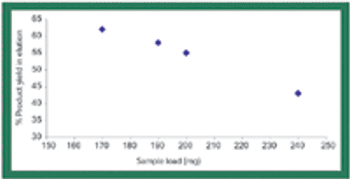
Rising drug costs have increased public pressure on the biopharmaceutical industry to find ways to identify and eliminate high-cost unit operations. Biopharmaceutical manufacturing groups now routinely evaluate both productivity as well as economic feasibility for every process step.
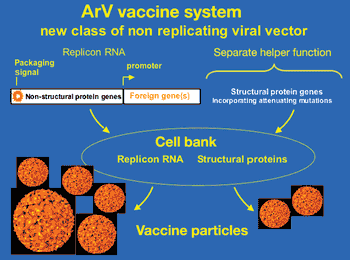
Vaccine developers are using novel drug delivery methods that offer advantages over traditional techniques such as improved immunogenicity, better stability, specific control over antigen release, and a wider pool of targeted diseases.

Pharmaceutical Technology has joined with INTERPHEX to found GenerationNext, an award program to recognize emerging leaders in pharmaceutical science and technology.

Vaccine developers are wrking on new drug delivery systems that offer improved immune responses, better stability, and a wider pool of targeted diseases.

The unit-dose bar coding rule requires integrating many aspects of packaging design and control.

FDA is finalizing guidances and enforcing compliance with manufacturing standards as part of its efforts to ensure drug safety.

A newly developed software program transforms written SOPs for all required analytical method validation experiments into transferable automated templates, integrating individual activities and technologies under one platform.

New technologies and improvements to existing ones can reduce contamination risk in aseptic processing.

A ready-to-fill closed vial can improve aseptic filling quality and reduce process complexity.

Asieve or screener is an essential part of every pharmaceutical production process, particularly as product quality and integrity are so important. The use of a sieve gets rid of oversized contamination to ensure that ingredients and finished products are quality assured during production and before use or despatch.
Pure water is a raw material of particular importance to the pharmaceutical industry. Drinking water is the basis for the treatment of water for pharmaceutical applications; it is the starting point for the production of the various pharmaceutical water qualities, such as purified water, highly purified water and water for injection.

The complete elimination of human-derived contamination is possible only with the elimination of human intervention.

The appropriate barrier system should be selected using a logical, risk-based approach, with awareness of all the possible sources of contamination.

The FDA initiative —Process Analytical Technologies (PAT) — is slowly gaining momentum, creating a revolution in manufacturing and testing processes that aims to ensure product quality. Its growth will encourage faster testing techniques to bring analytical testing closer to on- and at-line testing during the product manufacturing process.
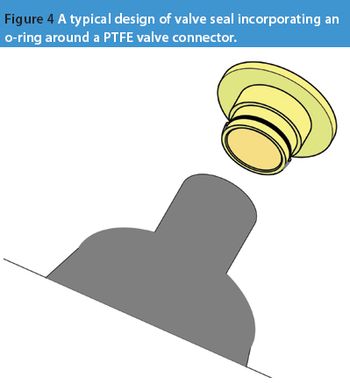
Active pharmaceutical ingredients (APIs) have become more potent, therefore the requirements of good manufacturing practice (GMP) are making ever more stringent demands on valve design and sealing. An absence of dead space, ease of cleaning and flushing is the norm for valves where cross contamination must be avoided at all costs. Sealing valves to glass reaction vessels has lagged behind valve sealing for steel vessels.
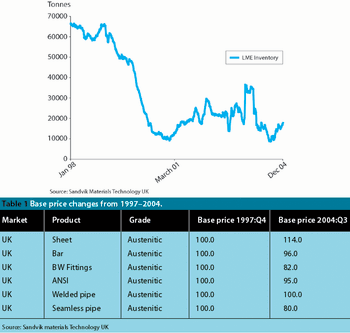
Improved products and processes that are cleaner, less expensive, more manageable and take up less space are benefits that you would expect to be snapped up by any industry. But not so in pharmaceutical manufacturing, where traditional methods prevail.

Study results show that the state of a cleanroom clothing system–new or much used–influences the protection efficacy of the system.