
Manufacturing
Latest News


December_Spotlight

KC News

Amid the finger-pointing, hand-wringing, and bloviating, the supply of hard, technical information remains frustratingly small.

This article provides guidance for minimally acceptable method validation practices and a foundation for assessing the risks and benefits associated with method validation programs.

A discussion of the validation and operation of two commercially available vapor-phase hydrogen peroxide decontamination systems is presented, based on a hands-on examination of both systems.

Pharmaceutical Science & Technology News

Pharmaceutical Science & Technology Innovations

With creative engineering and sophisticated chemistry, industry scientists offer big solutions for attaining small particle sizes and narrow distributions.
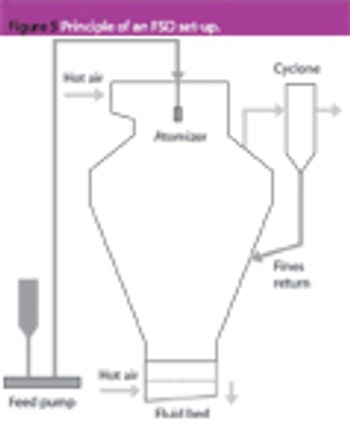
Granulation is one of the most important unit operations in the production of pharmaceutical oral dosage forms. However, there are many different technologies each having different strengths and weaknesses. Most companies choose which one to use simply based on their own experience. This article introduces different processes, compares them objectively and offers unbiased advice on the merits of each system. It then looks at the implications of selection on two different applications.

Packaging machines for new or existing lines are easier to operate and change over than they ever were before. Today's packaging machines also accommodate a greater variety of heights, diameters, finishes, or dosage regimen counts.

This article describes a novel approach to a scale-up management system, based on a holistic view of the scale-up lifecycle and an accompanying electronic development record of the information created.

The author argues that information technology solutions will play a major part in confirming-by experimental and statistical validation-that bioprocesses may be interchangeable and therefore generic. The bioprocessing ANDA will define both in-house and contract manufacturing.

Pharmaceutical science and technology news

Freeze pelletization is a new and simple technique for producing spherical pellets for pharmaceutical use.

The authors review current industry practices and regulatory expectations for the aseptic processing of sterile drugs. They compare and outline critical issues in current manufacturing technology and capabilities with regulatory requirements.

Stakeholders consider ways to improve printed patient inserts.

Using dimensionless analytical models, the authors establish the relationships among the essential parameters of a kneading complexation process.

By enhancing stability and in vitro drug-release characteristics, limonene enantiomers are a natural-source alternative for developing and characterizing self-nanoemulsified drug delivery systems.

Evaluations have shown, in most cases, visual observations are sensitive enough to verify equipment cleanliness. An experiment was conducted to explore the possibility of using a visible-residue limit as an acceptable cleaning limit in a pharmaceutical research facility, including an evaluation of the limits and subjectivity of ?visually clean? equipment.

Big Pharma is ramping up capital spending in parenteral manufacturing at the same time that contract manufacturers are completing their own major investment programs.

Transdermal drug technology specialists are meeting demands for methods that can painlessly deliver larger molecules in therapeutic quantities.

This paper discusses the most important factors that a filter user must take into account when making a filter selection.

This article discusses the basics of sterile filter qualification and validation, with emphasis on bacterial challenge protocol development and testing. Reference is made to Technical Report no. 26 of the Parenteral Drug Association.

Steaming-in-place (SIP) is a widely adopted method for the in-line sterilization of processing equipment. The main advantage of SIP relies on manipulation reduction and aseptic connections that might compromise the integrity of the downstream equipment.



