
Boehringer Ingelheim and and Hisoar (Taizhou, China), a pharmaceutical production company, formed a strategic production alliance in China.
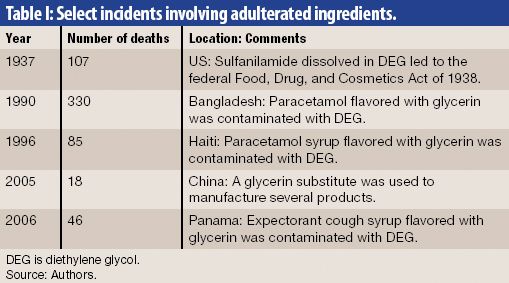

Boehringer Ingelheim and and Hisoar (Taizhou, China), a pharmaceutical production company, formed a strategic production alliance in China.

Also, Novartis stops development on "Aurograb," Zealand Pharma appoints David H. Solomon as CEO, more...

CMC service providers are doing well, but clinical and preclinical CROs are doing even better.

Brief pharmaceutical news items for September 2008.

This year, the employment survey will acknowledge the industry's best employers.

FDA is taking several measures to ensure that imported drugs meet manufacturing standards.

Summer deals struck by Novartis and Lilly point to an evolution in contract services.

Are hypersanitation trends a result of scaremongering or a lack of faith in medicine?

The outlook is fairly optimistic as contract manufacturing organizations (CMOs) gather at CPhI Worldwide in Frankfurt. CMOs are expanding capacity for small-molecules, biologics, and finished-product manufacturing.

All commercial sponsors want to maximize their profit and, with a population close to 500 million, the EU is an enormous potential marketplace that they simply cannot ignore!
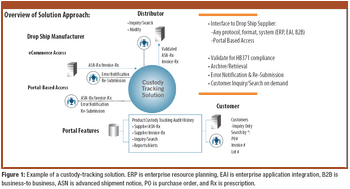
EPedigree, track-and-trace technologies, and other tools for optimizing supply-chain management are of increasing importance to the pharmaceutical industry. The author examines the current regulatory and legislative framework for ePedigree for finished drug products as well as proposals to require electronic statements for pharmaceutical ingredients.

The authors describe the critical aspects of an ideal fermentation services provider.

The Healthcare Institute of New Jersey (HINJ) released a new research study showing that New Jersey's life-science industry experienced stable growth in 2007.

The deadline for nominations for the Innovations in Pharma Science Awards is here. Check out http://pharmtech.com/Innovations to nominate your company's work in one of five areas by next week

Also, Shire recalls "Daytrana" patch, Chromatide forms Scientific Advisory Board, more...

Also, Tekmira will collaborate with Bristol-Myers Squibb, Helicos Biosciences appoints Steve Lombardi CEO, more...

The US Food and Drug Administration seeks contractors that will identify, describe, and evaluate potential data sources or data environments that could participate in the agency's Sentinel Initiative.

Also, Ortho Biotech recalls one lot of "Procrit" due to cracked vials, more appointments at Actavis, more...

The House Committee on Energy and Commerce issued a revised discussion draft to the Food and Drug Administration Globalization Act of 2008.

Also, Bilcare Global Clinical Supplies opens new headquarters in San Francisco, Sigurdur Oli Olafsson named CEO of Actavis Group, more...

The pharmaceutical industry?s outsourcing practices receive increased attention as Congress evaluates the role of outsourcing in drug-safety issues.

Providing the right information upfront may ease new requirements to assess solvent levels.

Strong profitability and good growth prospects bode well for the global market for contract research organizations.

CMOs expand capacity and capabilities in high-potency manufacturing to meet strong demand for cytotoxic and other potent drugs.

Legislative decisions to increase Medicare's formulary may lead to a fight over drug approvals.