
Company and People Notes: BASF raises prices on excipients; Verus Pharmaceuticals appoints president and CEO; more.

Company and People Notes: BASF raises prices on excipients; Verus Pharmaceuticals appoints president and CEO; more.

Pfizer's Lipitor patent revoked in Germany, promotions at Charles River Labs, more.

Recent activity among contract manufacturing organizations include expansions for Lonza and Codexis in Asia and the addition of aseptic cytotoxic capabilities for DSM. Also, Vetter adds anticounterfeiting capabilities for prefilled syringes.

Ash Stevens is adding cryogenic capabilities as part of an overall strategy to focus on small molecules requiring smaller volumes and complex chemistry.

Equipped with a new sourcing strategy in Asia and improving market conditions, Pfizer CentreSource expects a good performance from its steroid business this year and in 2008.

The US Food and Drug Administration launched a new program on Oct. 4 to increase the number and variety of generic drugs available to the public, beginning in fiscal year 2008. The Generic Initiative for Value and Efficiency (GIVE) will use existing resources to help the agency "modernize and streamline the generic drug approval process," according to FDA.
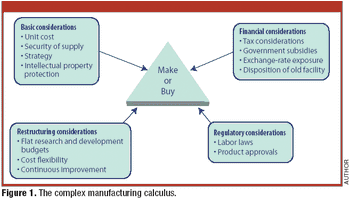
Candid comments from a Big Pharma executive highlight the complexity of contract manufacturing.

News and Views
Pharmaceutical manufacturers are under increasing pressure to shorten time-to-market, produce treatments with unpredictable product lifetimes, provide greater flexibility and, at the same time, comply with ever more stringent quality, validation, stability and traceability constraints. While this is encouraging for the contract manufacturing sector, it creates the need for even greater manufacturing flexibility.

Company Notes: Gibraltar expands facilities; Allozyne appoints new president and CEO, more.

Company and People Notes: ImClone, BMS, and Merck form agreement; CRI Worldwide names new CEO, more.

Company and People Notes: Orexo acquires Biolipox, Avalon cofounder resigns, more...

Company and People Notes: Novartis and MIT to study continuous processing, GSK appoints Andrew Witty as CEO, more.

Company and People Notes: Thermo Fisher Scientific buys Priority Solutions International, Wyeth elects new president and CEO, more.

The rising influence of Asia in the global pharmaceutical ingredients market was evident at CPhI Worldwide.

Restructuring among contract manufacturing organizations and suppliers of pharmaceutical ingredients this year is bringing a new group of faces to CPhI Worldwide this year.

Change is inevitable, as is contract manufacturing. But which companies will survive the drifts?

Could sloppy due diligence practices hurt CMOs and their clients?
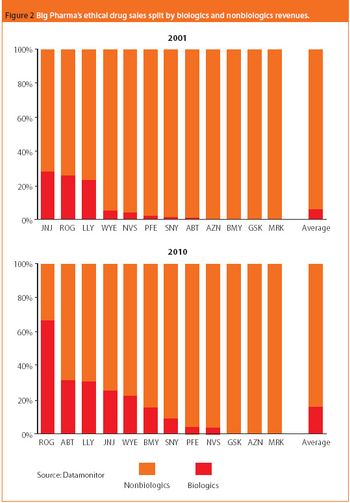
In 2007, the global pharmaceutical market is expected to grow moderately while biologics, generic drugs, and specialty-initiated drugs are projected to increase at double-digit rates. These trends for finished pharmaceuticals are reflected in the global market for APIs, where the merchant generic API market is expected to see strong demand. On a production basis, India and China are forecast to raise their shares of the global generic API market against industry strongholds Italy and Spain. Meanwhile, the US is expected to hold its its position as the leading producer of biotechnology-based APIs in an area traditionally dominated by captive production. And biogenerics or biosimilars gradually reshape the market.

Company and People Notes: Evotec and Renovis enter agreement, Amgen to lay off 675 workers, more.

Company and People Notes: Catalent expands Bolton, UK, warehouse; Biotica appoints Edward E. Hodgkin as CEO and director; more.

Sartorius has officially started up operations at its new plant in Beijing. By building the new facility, the company has now doubled its production capacity in China from approximately 4000 m2 to more than 8000 m2; an additional building phase makes it possible to even quadruple the floor space in the future. The investment volume for the new plant site is about ?10 million.

Company and People Notes: Baxter and Halozyme Expand Relationship, Crucell Names COO, More.
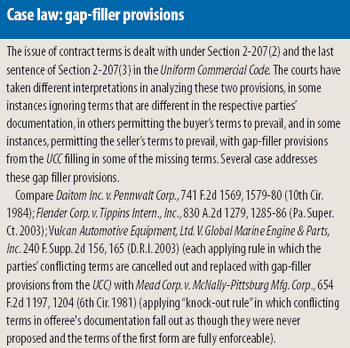
Purchase-order contracting is a commonly used approach to conducting commercial transactions, but it is a risky proposition when applied to pharmaceutical transactions, including the buying and selling of contract services and pharmaceutical ingredients. The authors examine the contract provisions covered in a commercial-supply agreement that are likely to be omitted under purchase-order contracting and the risk-reduction benefits that a commercial-supply agreement can offer in pharmaceutical procurement transactions.

Aptuit expands its drug-development capabilities with the formation of Aptuit Laurus to take advantage of the growing pharmaceutical outsourcing market in India.