
This article discusses fully automatic inspection of glass and plastic containers and factors that affect particle detection rate.

This article discusses fully automatic inspection of glass and plastic containers and factors that affect particle detection rate.

Die-cut strip packaging made with Romaco’s Siebler heat-sealing technology will be displayed at Constantia Flexibles’ booth (Stand 42H51) at CPhI Worldwide 2017.

The Gx Elite vials are the result of comprehensive optimization measures in the conversion process, which have focused on designing out the risk to create product flaws during production including the removal of all glass-to-glass contact beginning with the tubing material all the way through final packaging.

Bormioli Rocco Pharma, an Italian supplier of glass and plastic packaging, has launched its Delta-molded, type 1 glass vials in North America.

Material innovations enhance packaging for injectables.
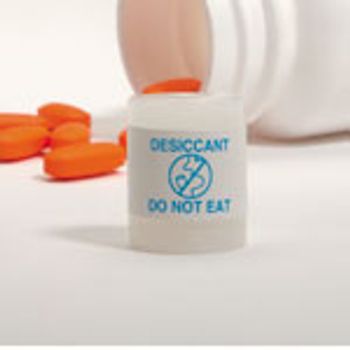
While cold-form, foil-foil (aluminumaluminum) blister packaging is considered the most moisture-protective packaging available, in fact, plastic containers such as bottles and tubes, when combined with desiccants, such as silica gel canisters and packets, will often provide lower internal relative humidity for long time periods.

The company is voluntarily recalling Lorazepam Oral Concentrate, USP 2mg/mL because of misprinted dosing droppers.

Sensors and communication capability support proper usage, improve compliance, and may enable telemedicine.

Cryopak’s CryoCube reusable temperature-controlled shipping systems are available in several insulation categories, and a tailored solution can be modeled using thermal modeling software.

Apace Packaging LLC labeling error initiates national recall of cyclobenzaprine HCL and amantadine HCL (Lot 16710).

Corning and Gerresheimer have been working together to accelerate innovations for pharmaceutical glass packaging.

A Merck, Pfizer, and Corning collaboration resulted in development of Corning Valor Glass for improved drug storage and delivery and will create US jobs.

A staggering percentage of people do not take their medication correctly, but pharmaceutical packaging aims to improve patient compliance using new technology to address reasons for non-adherence.

Gerresheimer’s glass and plastic prefillable syringes will come with an innovative, integrated, passive syringe safety solution, acquired through an exclusive licence from West Pharmaceutical Services.

The suite installation increases PCI’s Hay-on-Wye site’s serialization capability to support clients in advance of meeting the implementation dates of the European Falsified Medicines Directive.

A staggering percentage of people do not take their medication correctly, but pharmaceutical packaging aims to improve patient compliance using new technology to address reasons for non-adherence.

In a new research conducted by SEA Vision and Zenith Technologies, 28% of respondents identified technology selection as the biggest challenge as the industry prepares to meet serialization deadlines.

Robotic fill/finish systems reduce human intervention, improve flexibility, and allow more gentle handling of containers.

Aptar now manufactures its child-resistant pump, designed to meet US CPC requirements, at its facility in Congers, New York.

Follow guidelines for E&L studies of an orally inhaled and nasal drug product formulation in its delivery device.

The company recalled the tablets due to a packaging error.

The new storage and distribution facility provides PCI with additional space, complementing its existing footprint currently used for specialist clinical-trial logistics as well as packaging, labeling, and qualified person activities for investigational medicinal products.

At INTERPHEX 2017, exhibitors displayed packaging innovations designed to improve quality, security, and patient protection.

The company is expanding their April 2017 voluntary recall of phenobarbital tablets.

Robotic fill/finish systems reduce human intervention, improve flexibility, and allow more gentle handling of containers.