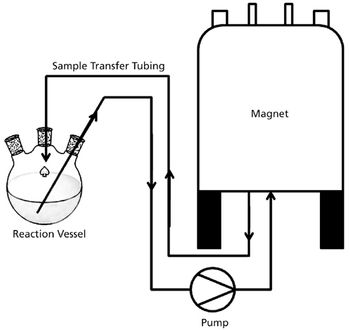
The authors describe the benefits of nuclear magnetic resonance (NMR) compared with traditional monitoring techniques. They also discuss how NMR reaction monitoring provides a new process analytical technology tool for industry.

The authors describe the benefits of nuclear magnetic resonance (NMR) compared with traditional monitoring techniques. They also discuss how NMR reaction monitoring provides a new process analytical technology tool for industry.

Industry buy-in is increasing as pharma companies proceed with select projects and research.
A perspective from Pfizer on the lessons from small-molecule manufacturing that can be applied to biomanufacturing.

Quality management requires more effort in a complex supply chain.

Pharmaceutical Technology's annual survey on equipment and machinery reveals the spending levels and type of spending made in 2010 and planned for 2011.

Industry and regulatory experts discuss the challenges and benefits of implementing real time release testing in a pharmaceutical manufacturing environment.

A look at MVI's malaria work in developing countries.
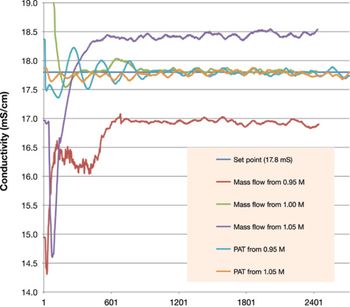
The authors describe the operational requirements and design of a process-ready PAT-based IBD system.

Leading experts share insight on the current and future direction of process analytical technology. This article contains bonus online material.
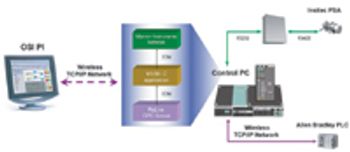
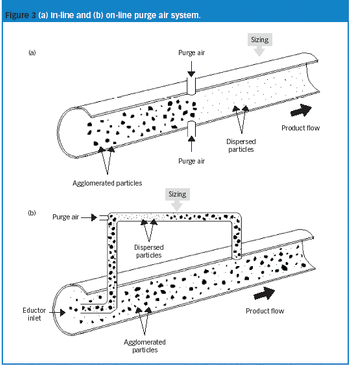
The authors describe the implementation of an on-line particle-size analyzer on an active pharmaceutical ingredient milling operation at a commercial site.

The author discusses control strategies via near infrared instrumentation for continuous mixing, granulation, drying, and extrusion with a more focused detail on mixing.

Robotic systems provide flexibility and efficiency (and they're not as difficult to use as you think). This article contains bonus online-exclusive material.
The author provides a review of PAT and tools such as near infrared analysis that may facilitate the use of PAT in the biopharmaceutical sector.
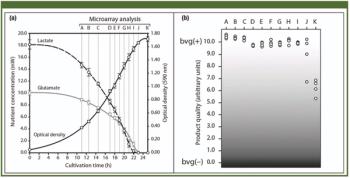
This case study describes the implementation of process analytical technology on the cultivation process step of a whole-cell vaccine against whooping cough disease.

The authors review the role of automation in aseptic processing and describe their experience in implementing advanced technologies, including the use of isolators and robotics.

The role of automation suppliers is transforming to meet the pharma industry's demand for change.
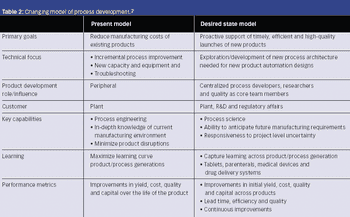
Operational excellence awaits, but only if you can implement PAT successfully.
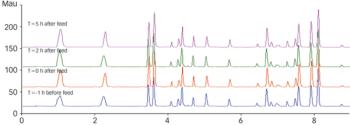
PAT guidance has been available from FDA for more than 4 years, but there have been no apparent breakthroughs in large-scale upstream production. Will companies consider using on?line chromatography to change this?
FDA advocates building quality into a product through PAT.

The biggest benefit of PAT and the current FDA initiatives may be in allowing pharmaceutical scientists to use their technical capabilities to improve pharmaceutical processes.

Getting IT, engineering, and manufacturing on the same page requires a delicate balance.

Enterprise process control and management (EPCAM) is a new strategy for healthcare manufacturers based on recent process-control breakthroughs in the electronics industry.

ISA 100.11a and WirelessHART both seek to become the global standard for industrial wireless automation.

A manufacturing line can be improved if technology transfer is implemented thoughtfully. Effective technology transfer helps to provide process efficiency and control and maintain product quality.

Six sigma, process analytical technology (PAT) and related initiatives are driving greater use of statistical analysis methods to increase process understanding and improve manufacturing capabilities.