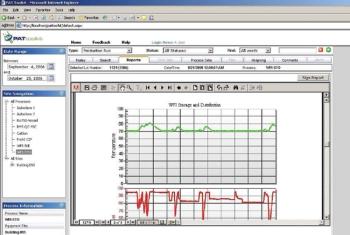
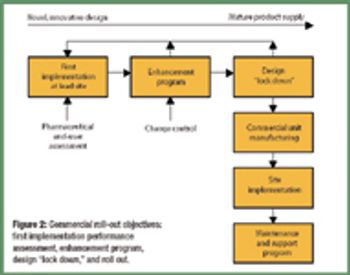
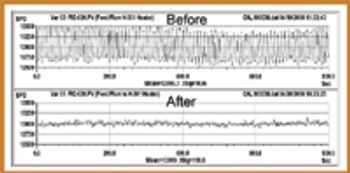
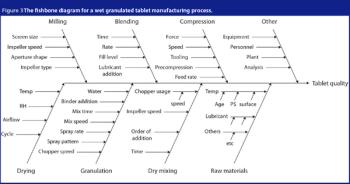
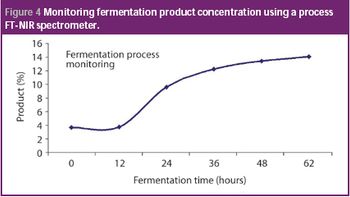
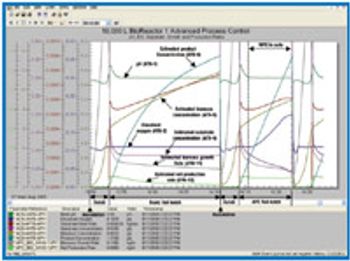
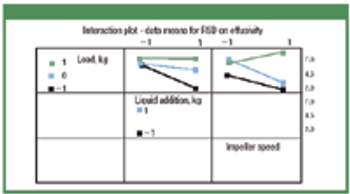
Automation took hold gradually in the life-science industry. Its adoption brought the industry innovations and improved efficiency. Recent years witnessed the emergence of batch-automation systems and the development of standards for automation. The authors discuss the major changes automation brought to the industry and examine the rapid pace of technological development.