
Agents report unusual chemistry, abnormal data analysis, and unconventional work practices.

Agents report unusual chemistry, abnormal data analysis, and unconventional work practices.

They may have seemed harmless at first, but these ideas took a big bite.

At first glance, the title of this article may bring a wry smile to the face of many an astute practitioner, but I can provide 'documentary evidence' that free validation is not a just a play on words, but a financial reality.

How can any company be sure that the standards that suppliers might claim to operate, and might be able to demonstrate from time to time, are actually being practised all the time?

Customers complaining lead to some serious explaining.

WFI system deficiencies and damp tax records cause problems for two plants

The assembly of the World Health Organisation (WHO) later this month is expected to feature counterfeit medicines as one of its important discussion points, and there is some hope that the ministers will agree on measures that will strengthen anticounterfeiting legislation and enforcement worldwide.
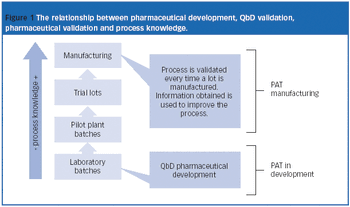
When Pharmaceutical Technology Europe was established 20 years ago, PAT was not a hot topic in the industry. It was started in 2002 by FDA to modernize pharmaceutical manufacturing and increase the efficiency of manufacturing processes.
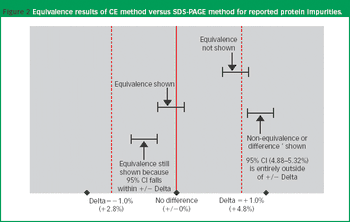
Practical guidance on how to handle validation failures cannot be found in the existing literature because they are not supposed to happen.

A manufacturing line can be improved if technology transfer is implemented thoughtfully. Effective technology transfer helps to provide process efficiency and control and maintain product quality.

Essential components of a good inspection: good ingredients, proper inserts, and ... deer?

Most CROs are entering China with expectations that a significant local market opportunity will develop as major pharmaceutical companies establish research development operations there...

Manufacturing facilities must be inspected by members of regulatory bodies. However… these bodies are woefully inadequate at performing the task.

If not properly monitored, filters and plastic bags can keep back more than they should.

If at first the product fails, then inspect, inspect again

Process efficiency is measured not only by what is kept, but also by what is thrown away.

The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) recently closed consultation on its draft guidance, ICH Q10 Pharmaceutical Quality System. If all goes to plan, adoption could come as soon as Spring 2008.
Historically, the main purpose of laboratory information management systems (LIMS) has been to track and manage samples in the laboratory. LIMS originated nearly 30 years ago as a rudimentary method of automating manual, error-prone processes in the laboratory and, with the growth in adoption of technology, became the de facto benchmark for laboratory control and management.

Getting a clear view of business performance can be cumbersome, time-consuming and even nigh-on impossible.

Chi-wan Chen, deputy director of the Office of New Drug Quality Assessment (ONDQA) at the US Food and Drug Administration's Center for Drug Evaluation and Research, shares insight from FDA's pilot program that was designed to allow pharmaceutical companies to submit CMC information demonstrating application of quality by design.

The "why" things are done in this industry is just as important as the "what"and "how."

Incorporating quality and economy into downstream purification processes can expedite first-in-human clinical trials, product licensure and technology transfer. Experience in the chromatographic purification of biopharmaceuticals enables the use of downstream processing heuristics to produce target molecules in cost-effective processes suitable for regulatory scrutiny.
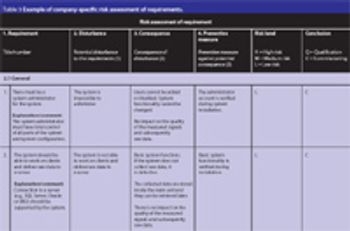
It is becoming evident that quality risk management within regulated, life sciences environments is a valuable component of an effective quality management system (QMS). A QMS provides a proactive and systematic means to identify, analyse, evaluate and control potential process and product quality issues during development, manufacturing, distribution and marketing throughout the entire product life cycle.

A well-devised QPP, which has been agreed on and signed by both parties, saves time and makes it easier to complete activities such as design, installations and tests.

Never has greater pressure been applied to pharmaceutical manufacturers. Shelf space competition for branded drugs has reached aggressive proportions and now even prescription drugs vie for pricing and delivery. Against this is a backdrop of ever-increasing downward price pressures, and a spectrum of progressively more stringent legislative and quality requirements. Finally, regional markets now demand different tamper evidence technology, anticounterfeiting measures and safeguards against interference by biological terrorists. Much of which points to the need for innovation in packaging - not just in terms of pack styles and sizes, but also cost.