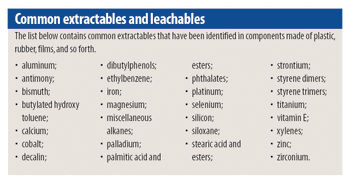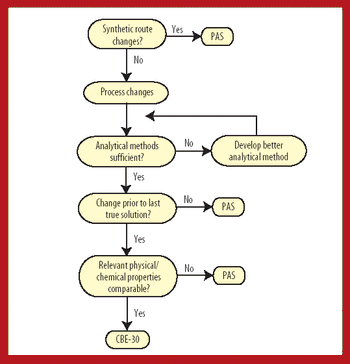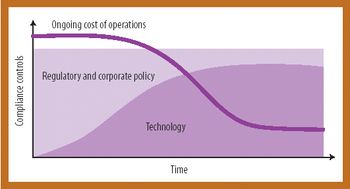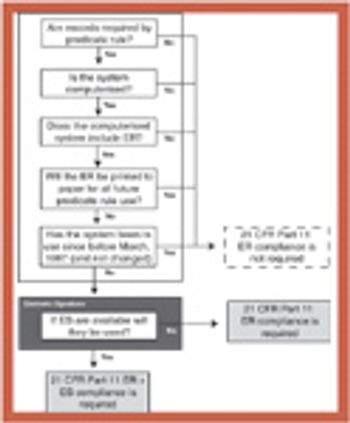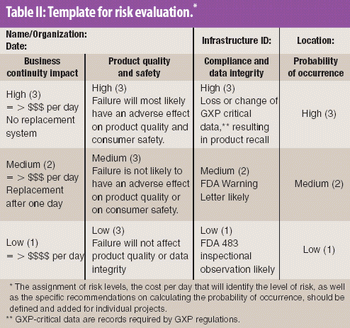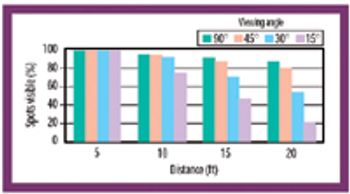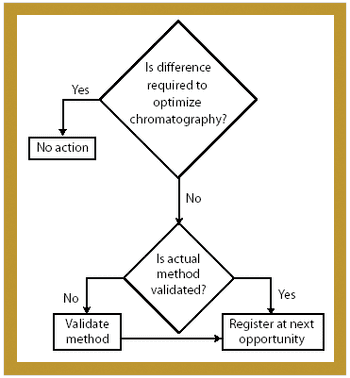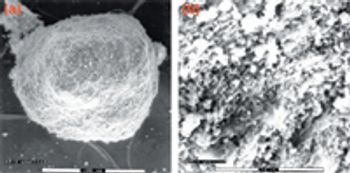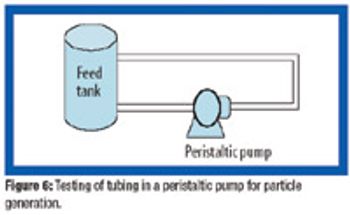
Because of the growing popularity of single-use materials, the identification, characterization, and qualification of new materials used for disposable processes have become increasingly important for both regulatory and production purposes. This article describes one approach to identifying and validating the materials used in a disposable filling process.