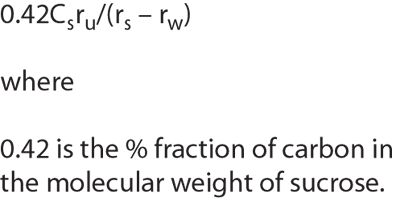The author argues that information technology solutions will play a major part in confirming-by experimental and statistical validation-that bioprocesses may be interchangeable and therefore generic. The bioprocessing ANDA will define both in-house and contract manufacturing.