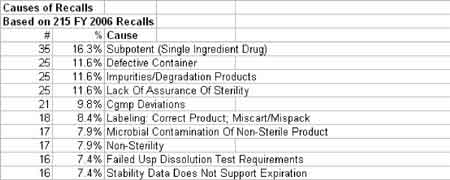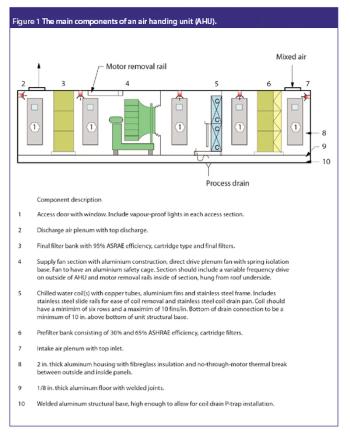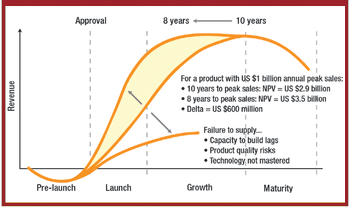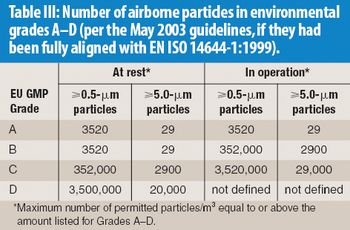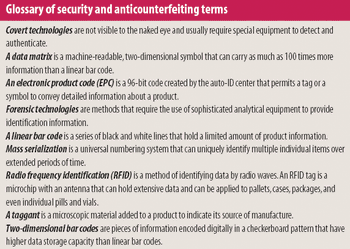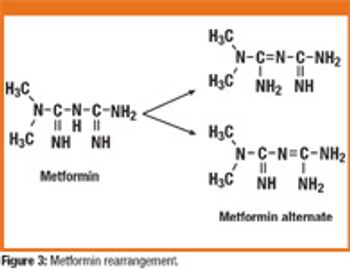
The correlation between swab assay results and visible-residue limits (VRLs) for cleaning validation was examined. Previously completed validation studies were reviewed to compare swab results with recently determined VRLs. A current cleaning validation study evaluated both swab testing and VRL. Unexpected swab results led to an investigation, which showed the value of establishing the VRL in conjunction with swab recoveries for cleaning validation programs.