
FDA sent a warning letter to Emcure Pharmaceuticals, Ltd. because of sterility testing CGMP violations.

FDA sent a warning letter to Emcure Pharmaceuticals, Ltd. because of sterility testing CGMP violations.

The company is recalling the product because of potential microbiological contamination.

Spectrum Laboratory Products received an FDA warning letter after violations were found at its New Brunswick, NJ facility.

Ridge Properties LLC DBA Pain Relief Naturally is voluntarily recalling four lots of 4% lidocaine topical cream and liquid gel products due to microbiological contamination and incorrect potency.

Quality risk management plans provide identified actions to ensure a continuous supply of safe and effective drug products, says Susan J. Schniepp, executive vice-president of post-approval pharma and distinguished fellow, Regulatory Compliance Associates.

Harmonization of regulatory guidelines not only reduces workloads for manufacturers and regulators but can potentially help to accelerate patient access to vital therapies.

Instrumentation advancements over the past 30 years have certainly enabled greater efficiency in pharma development and analysis despite slow adoption by the industry.

To deal with the complex requirements of biopharmaceuticals, companies need a sophisticated toolbox of analytical and purification techniques.

European Union regulators have taken a significant step towards resolving some of the major supply-chain difficulties behind medicine shortages.

Machine vision systems have been an integral part of pharma manufacturing and packaging for many years and, with the introduction of stricter safety regulations, are set to become more vital.

Strong personnel training, detailed SOPs, commitment to data integrity, investigation and implementation of appropriate modern methods, and employing Lean and Six Sigma methodology initiatives are key best practices for the quality control microbiology lab.

The ZipChip CE-ESI interface was evaluated for suitability as a platform approach for quantitation of MIs in API.
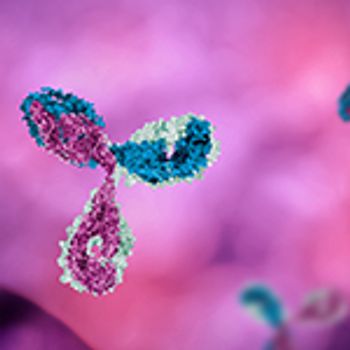
Protein characterization is a critical part of drug development, but as there are still limitations with available techniques, industry needs to look at technological advances to meet the specific requirements of complex molecule characterization.

Senior managers of OTC drug companies are on a learning curve, as FDA warning letters cite insufficient understanding of cGMPs and inadequate responses to prior 483s.

A July article in Science suggests that FDA enforcement activities have dropped significantly during the current US presidential administration.

Altaire is voluntarily recalling ophthalmic products because of concerns regarding quality assurance controls in the manufacturing facility.

FDA sent a warning letter to Ecometics, Inc. after the agency found CGMP violations at the company’s Norwalk facility.

The draft guidance describes how content should be organized in electronic submissions for all submission types under section 745A(a) of the FD&C Act.

Slovakia becomes the final European Union country to be recognized by FDA, and the mutual recognition agreement for inspections of manufacturing sites between the US and the EU is now fully implemented.

The FDA guidance defines changes to approved risk evaluation and mitigation strategies and clarifies submission guidelines.

The draft guidance provides industry with a guide for using the database to assist in the development of drug products.

FDA released draft guidance on using the USP pending monograph process in the drug application process.

FDA sent warning letters detailing violations of current good manufacturing practices to three companies that repack APIs.

Cultural and language discrepancies during an audit can be resolved using what many call a “playbook,” says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.

Today’s inspection systems catch tinier flaws, manage data, and increasingly rely on artificial intelligence to further boost performance.