
A required time frame should not be the driving force behind root cause investigations, says Susan Schniepp, executive vice-president of Post-Approval Pharma and Distinguished Fellow, Regulatory Compliance Associates.

A required time frame should not be the driving force behind root cause investigations, says Susan Schniepp, executive vice-president of Post-Approval Pharma and Distinguished Fellow, Regulatory Compliance Associates.
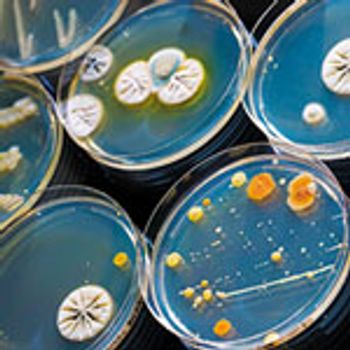
Microbial identity data can be critical for determining contamination sources.

Success depends on supplier communication and transparency, but it’s up to buyers to demand the right information and to look at the vendor’s overall business goals.

Simplified role-based training can lead to better quality metrics and compliance.

Once described as “throwing processes over the wall,” tech transfer is evolving into close collaboration and communication, as potential problems are considered sooner, and new technology is applied. Joseph Szczesiul, director of technical services for UPM Pharmaceuticals, shares best practices.

Drawing on practical experience, the authors examine key questions and answers about various aspects relating to the enhanced approach for analytical procedure lifecycle management.

A new, high-throughput microplate reader cuts down on screening time and works faster than standard ultra-high-performance liquid chromatography processes.

The partnership, co-funded by Enterprise Ireland, will develop technologies for monitoring the quality of biopharma processes.

FDA has issued a warning letter to Mylan citing GMP violations of finished pharmaceutical products manufactured at the company’s Morgantown, WV, facility.

The agency sent a warning letter to the company for marketing an unapproved stem cell product and CGMP violations.

The agency is developing a new way to assess, record, and report data from surveillance and preapproval inspections of sterile drugs.

An ERP solution provides for the management of multiple business activities and traceability requirements resulting from regulations, customer demands, sourcing, and international business needs.

Building up relevant expertise in-house will make writing spec sheets for software easier, according to Siegfried Schmitt, principal consultant at PAREXEL.

This paper describes how the concept of acceptance value can be redefined to remove bias and more closely reflect quality targets.

Experts share best practices, and war stories, for a crucial but often underappreciated part of drug development.

Mock inspections-if conducted properly-can prepare a pharma company for the day FDA knocks on the door.

Takhzyro (lanadelumab) is the first monoclonal antibody therapy approved for the prevention of recurrent attacks of hereditary angioedema (HAE) in patients aged 12 years and older.

Promise Pharmacy recalls one lot of Prednisolone and Gatifloxacin Ophthalmic Solution 1%/0.5% sterile, 3mL vials due to particulates in the solution.

Experts blame the recalls, not on cGMP failures, but on inadequate risk assessment of processes that can generate toxic impurities.

Susan Schniepp, executive vice-president of Post-Approval Pharma and Distinguished Fellow, Regulatory Compliance Associates, takes a look at the regulations around data integrity and how they relate to the concept of quality culture.

A European task force outlines its upcoming efforts to combat drug shortages.

This pharma insight briefing from Agilent Technologies will cover the application of Raman and infrared spectroscopy for pharmaceutical quality control (QC).

The agency sent a warning letter to the Indian company after an inspection found CGMP violations that included a lack of written procedures and analytical testing.

The agency announced that differences in strength expression on product labels of compounders and conventional manufacturers may lead to dosing errors.

Cell-line quality has a significant impact on biologic drug quality; learn more about this and other upstream challenges at the new bioLIVE launching this year adjacent to CPhI Worldwide 2018.