Shortages and compounding disaster spur efforts to overhaul manufacturing oversight and stimulate industry improvements.

Shortages and compounding disaster spur efforts to overhaul manufacturing oversight and stimulate industry improvements.
A change in terminology could emphasize patient protection.

Real-time experimentation may offer continuous process improvement.

Identifying and classifying rouge can help to determine CAPA.

Applying the recommendations of ICH Q10 to statistical analysis can help prevent product recalls.

Experts share insights into analytical tools and techniques.

Applying current principals to traditional factorial designs.

Uniform dose formulation is key to meeting safety study requirements.

Are pharmaceutical manufacturers really serious about ensuring the quality of their medicines? And do they recognize that cutting corners on controls and quality management can be tremendously costly in the long run?

Understanding the differences between convenience, target, and self-selected samples.
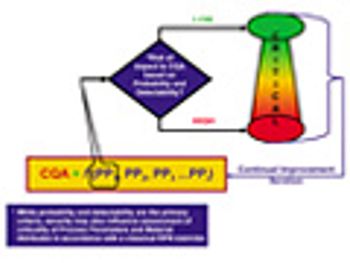
The authors provide an overview of the new ISPE Guide Series on Product Quality Lifecycle Implementation and how the guides can be used in a complementary way with existing guidance from FDA and the International Conference on Harmonization.

The authors detail the possible consequences of noncompliance and a lack of quality control.

CML - Quality Assurance
Where is the variability coming from and what have we done to minimize it?

Precedents set in the historic Barr case continue to raise questions over suitable sample-size criteria.
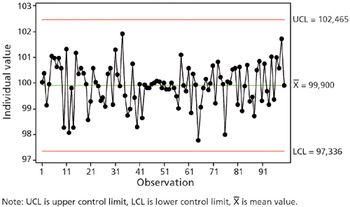
Current GMPs demand full understandng of out-of-control concepts. This article contains bonus material.
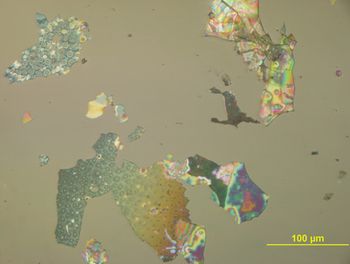
Glass flaking or delamination can result in a failed quality-assurance test, thus bringing production to a halt and causing substantial revenue loss. If glass delamination remains undiscovered, it can pose a serious contamination risk to the drug product and a potential health risk to the public.

A summary of the latest steering committee and expert working groups' meeting.

A firm grasp of probability and ongoing re-evaluation are key.

A book about developing quality-control training manuals provides a wealth of information and a dearth of practical help.
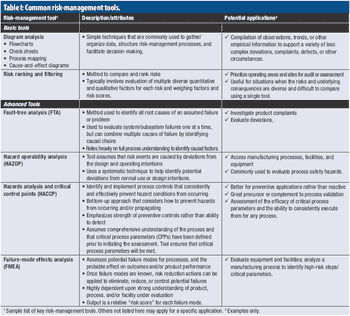
A PQRI expert working group provides case study examples of risk-management applications.

Ensuring compliance though increased statistical knowledge and resources.

The hardest errors to spot are the ones that don't look like errors at all.

The United States Pharmacopeial Convention (USP) launched a 12-month pilot Technical Assistance Program (TAP) to provide developing countries in sub-Saharan Africa with greater capacity to test the quality of medicines.

Using risk assessment properly can provide industry with a unique tool for quality control.