
Instruments are introduced for elemental impurity analysis and materials identification.

Instruments are introduced for elemental impurity analysis and materials identification.

Non-destructive container closure integrity testing allows 100% inspection of all ampoules, syringes, vials, and cartridges.

Industry experts and FDA’s Office of Pharmaceutical Quality discuss the challenges, trends, and regulations involved in ensuring quality in solid and semi-solid dosage forms.

Understanding of the risks associated with FMEA is crucial in lot release testing.
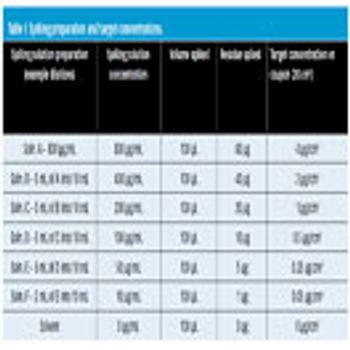
Visible residue limits have been shown to be a valuable tool in validated cleaning validation program.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss how to create a robust CAPA system and how to identify root cause.

Transparent communications, both qualitative and quantitative data, and a clear understanding of each other’s needs are keys to collaborating on better product quality.

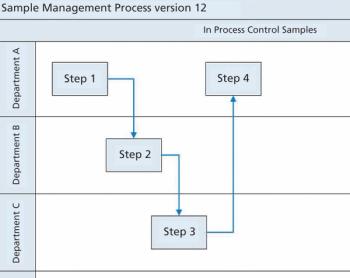
Siegfried Schmitt, principal consultant, PAREXEL, discusses how to streamline the document management process during market expansion.

While stakeholders generally welcome improvements to quality initiatives, they are concerned with how the new requirements will be implemented for more complicated supply-chain models.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to assure compliance for automated systems.

FDA’s proposed guidance for quality metrics raises questions about quantifying the tangibles and intangibles of quality culture.

QbD is improving the safety of solid-dosage drug products as well improving manufacturing processes, despite some industry reluctance.

To enable efficient monitoring systems, life-science companies need to effectively apply run rules.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general council, both of Regulatory Compliance Associates, discuss the requirements for a successful corrective action and preventive action (CAPA) system.

Mitigating risk in the bio/pharmaceutical sector demands a holistic approach.

Checkweighers, metal detectors, x-ray inspectors, leak detectors, headspace analyzers, and optical inspection systems for packaging were demonstrated at INTERPHEX 2015.

Sepha's VisionScan Max can leak-test full production batches of blister packs.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to handle internal audit reports during inspections.

Heightened regulatory scrutiny of data integrity highlights the need for comprehensive procedural reviews and strategies for managing mission-critical information.

An ISPE guidance document, four years in the making, brings risk-based thinking, statistics, and Lean Six Sigma to cleaning validation.

Presenters at IVT's Microbiology Week discussed best practices and recent guidance publications for microbial control in sterile and non-sterile pharmaceutical processes.

Optical inspection equipment for packaging was displayed at INTERPHEX 2015.

The new Kaye Validator AVS combines accurate sensor measurements with all GMP requirements for calibration and traceability.

The basic principle behind QbD is that quality should be built into a product with an understanding of the product and process by which it is developed and manufactured along with a knowledge of the risks involved in manufacturing the product and how best to mitigate those risks.