
The new document answers questions regarding implementation of the ICH Q7 guidance on GMPs for APIs.


The new document answers questions regarding implementation of the ICH Q7 guidance on GMPs for APIs.

The European Pharmacopoeia Commission has approved a general monograph laying down harmonized requirements for LBPs for human use and two general chapters addressing microbiological contamination of LBPs.

A new report gives an overview of the work of the International API Inspection Program.

Premier Pharmacy Labs is voluntarily recalling multiple products because of the potential lack of sterility assurance.

A new book by Robert Thomas, principal consultant at Scientific Solutions, provides a training tool for novices and inexperienced users of plasma spectrochemistry as well as for supervisors and senior management who want to better understand the analytical issues. Measuring Elemental Impurities in Pharmaceuticals: A Practical Guide, published on Feb.




The company is recalling Pasta De Lassar Andromaco zinc oxide diaper rash treatment after FDA analysis confirmed the product contained high levels of yeast, mold, and bacteria.

Implementing electronic batch records can offer a means of compliance, reduction in errors, streamlined ability to trace actions, and simultaneous generation of documentation.

Telstar reports that its Boreas, a new 86° ultra-low-temperature freezer, increases average performance by 20%.

The agency sent a warning letter to Malladi Drugs & Pharmaceuticals Limited after an inspection found CGMP violations that included the presence of vermin.

The delivery device and drug form should be considered when choosing a test method for identifying and measuring particulates.

A previously published article presented difficulties with the revised European guidelines on sterile manufacturing. The authors included a brief summary of the comments developed on the draft document. This article expands upon that summary, outlines the authors' rationale, and highlights the most difficult aspects of the revision draft.
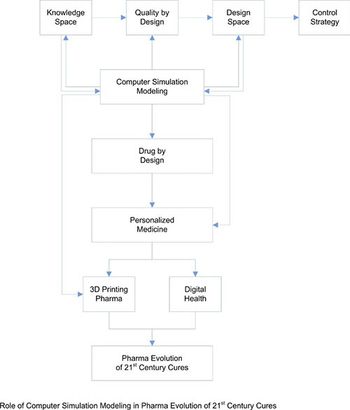
Twenty-first century drug development challenges sponsors and contract partners to collaborate more closely, using tools such as model-based simulation.

The company is recalling Methylprednisolone Sodium Succinate for Injection, USP, 40mg, 125mg, and 1g manufactured by Gland Pharma Ltd and distributed by Sagent due to out-of-specification impurity results.

The company is recalling three lots of Hydromorphone HCl Injection USP CII 10 mg/mL, 1 mL in 2 mL Single Dose Vials because of possibly empty or cracked vials.

In-house experts can help select the right systems and suppliers, making validation and compliance easy, says Siegfried Schmitt, principal consultant at PAREXEL.

Robert Iser of PAREXEL Consulting answers questions regarding the regulatory expectations of quality agreements and how companies can ensure the quality and safety of their products.

Stability testing on APIs/finished drug product helps define optimal drug packaging for shelf-life storage.

The company is voluntarily recalling three lots of Labetalol Hydrochloride Injection, USP, 100 mg/20 mL Vial and one lot of Labetalol Hydrochloride Injection, USP, Novaplus because of the potential of cracked glass at the rim of the vials.

The following study describes a methodology to establish, control, and characterize an aerosolized bioburden within a test chamber and determine the settling rate of the bioburden.

The NECC supervisory pharmacist at the center of the 2012 fungal meningitis outbreak was sentenced to 8 years in prison.
Regulations and industry guidelines focus on ensuring excipient safety by specifying risk assessments and shared responsibility.

While more companies are embracing taggants, researchers are developing new technologies that will be extremely difficult to reproduce.