
When it comes to getting the best out of quality by design, timing is everything.

When it comes to getting the best out of quality by design, timing is everything.

Pilot tests will evaluate a system developed by Ambrosus Technologies, using smart contracts and advanced sensors to ensure pharmaceutical quality, safety, and traceability.

The review of batch records creates a story of the materials, manufacturing, and packaging involved in the production of bio/pharmaceuticals, according to Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

Investigational failures and discrepancies can be avoided through the proper execution and documentation of investigations.

Weak or faulty quality systems can hurt a company at every stage of a product’s lifecycle.

One day after Ocular Therapeutix sent FDA a detailed response highlighting manufacturing improvements, FDA rejected its application for Dextenza, a new drug designed to alleviate post-operative eye pain.

This article presents a general strategy for authorship of deviation investigations, with primary focus on regulatory inspection success.

FDA’s process validation guidance has evolved and the current lifecycle approach has profoundly influenced validation practice.

Greater transparency and reliability of information are needed in the quality assessments of biosimilars.

Fagron Sterile Services has voluntarily recalled three lots of Succinylcholine Chloride 20mg/mL 5mL syringe to the hospital/clinic level.

FDA sent a warning letter to drug compounder DCA, Inc. dba Beacon Prescriptions for failing to ensure sanitary conditions.

Informing your clients of possible changes in equipment is imperative when upgrading a laboratory, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

Archana M. Akalkotkar, PhD, research scientist I at Charles River, discusses the use of process validation to ensure quality.

A multi-pronged approach to raw materials testing can help mitigate the risk of future contamination events.
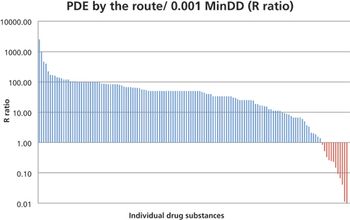
In this study, the authors investigated the relationship between the 0.001 MinDD and the PDE values for 140 drug substances as an attempt to identify high-risk groups of products for patient safety. This comparison can serve as a method for prioritization of APIs for development of PDEs.

Truxton, Inc. is voluntarily recalling one lot of Phenobarbital Tablets, USP, 15 mg because of a labeling error on declared strength.

The complex nature of biologics adds additional CQAs that must be determined to ensure the safe development of biologics

Pharmaceutical Technology asked Siegfried Schmitt, principal consultant at PAREXEL, about the importance of quality agreements in the sponsor/contractor relationship.

More life-sciences companies are starting to manage global suppliers holistically.

By working together and taking a QA-based approach, manufacturers and suppliers can reduce raw material testing requirements.

As pharmaceutical quality metrics evolve, they will need to incorporate more of the principles of operational excellence, says consultant Prabir Basu.

Could greater market transparency improve pharmaceutical quality and regulatory compliance?

The new facility will focus on formulation development, drug product analytical development, and quality control.
Outcry against high pharmaceutical pricing brings questions of high R&D costs and restrictive payers, but also drug development and manufacturing efficiency.
The media blitz surrounding drug shortages has stopped, but critical medications that have no substitutes remain in short supply. Can new approaches turn this situation around?