
A new machine is designed for inspection of prefilled syringes.

A new machine is designed for inspection of prefilled syringes.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses good engineering practices.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to handle staff challenges to regulation requirements

The author proposes a quality management system that uses the power of executive management to promote a positive quality culture.
The parenteral manufacturing industry is taking action to address particulate contamination issues.

A selection of articles and news about quality metrics.

After almost two years of anticipation, Janet Woodcock, director of the Center for Drug Evaluation and Research (CDER), has administration approval for organizational changes to bolster programs and policies to ensure drug quality.

The European Medicines Agency releases highlights from its Pharmacovigilance Risk Assessment Committee safety review meeting in October.

We?ve just opened new pharmaceutical laboratories! Our experienced scientists offer independent chemical and microbiological quality control testing of APIs, raw materials, finished products and medical devices in compliance with cGMP

FDA report details risk mitigation projects.

PDA surveys are designed to evaluate quality metrics practices at member organizations.

Cubist Pharmaceuticals voluntarily recalls certain lots of CUBICIN 500 mg in 10 mL single-use vials because of the presence of particulate matter.

Biopharma manufacturers must reduce the risk in their complex supply chains
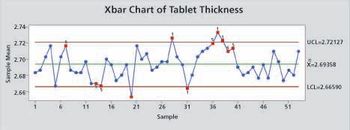
Control charts that are properly constructed and maintained prevent false out-of-control signals and provide a useful method for monitoring a process.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to ensure data integrity.
Statistical analysis shows how much testing is needed to deliver a reliable estimate result.

A drug sponsor?s responsibility does not end when a task is outsourced.

Life-sciences companies can get a jump on potential quality problems by looking at the leading indicators hiding in the document management process further upstream.

Industry players offer suggestions for quality metrics as FDA continues to try and solve the problem of drug shortages.
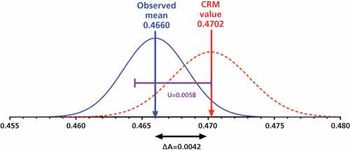
This article takes a statistical look at the calibration requirements for a UV spectrometer.

PharmaCheck, a portable tool designed to detect poor-quality medicines, has been selected by Scientific American as one of the magazine?s World Changing Ideas of 2013.

An operational-excellence initiative increased collaboration between operations and quality personnel to address human-error-caused deviations.

Siegfried Schmitt of PAREXEL discusses regulation requirements for quality systems.

Recent FDA enforcement activity reveals issues with vial-filling, adequacy of QA/QC procedures, particulate matter in inhalation powders and injectables, and drug labeling.
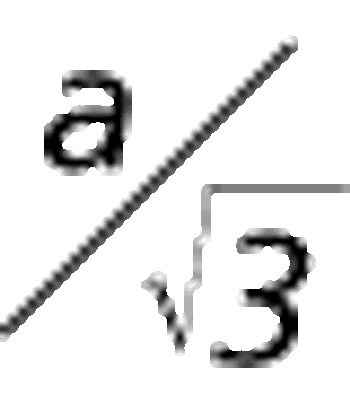
How good is a reportable value?