
The US and EU move forward with measures to fortify the pharmaceutical supply chain.

The US and EU move forward with measures to fortify the pharmaceutical supply chain.

Upcoming requirements in the US and around the world for serialization and track and trace of pharmaceuticals were a focus of the Pharmapack conference held in Philadelphia, PA earlier this week.

Bills to regulate drug compounding and establish a national track and trace system face political and policy differences.
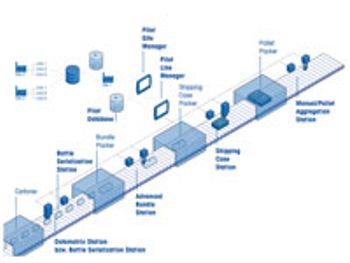
Integrated serialization systems keep pace with industry demand.

New Center for Pharmaceutical Advancement and Training increases number of experts and available tools in Sub-Saharan countries.

Pfizer has launched a prescription-fulfillment website for Viagra tablets in an effort to combat the online sale of counterfeited medicine.

Drug companies team up with INTERPOL to keep counterfeit medicines off the Internet and out of the hands of patients.

FDA will use a new anticounterfeiting tool to detect fake medicines.

EMA has upgraded its EudraGMP database to include information on GDP in addition to GMP. The new EudraGMDP database is a key deliverable of the Falsified Medicines Directive (FMD) that came into effect in January 2013. The aim is to increase supply chain security in the EU by making supervision of manufacturing and distribution of medicines more robust to ensure supplier compliance.

Mary Storch, a member of the planning PDA supply chain committee, is associate director, external QA oversight, for Ben Venue Laboratories. She discusses parenteral drug-supply chain challenges, in terms of the complexity of the raw materials supplied as well as additional consideration in end-product distribution, such as cold-chain requirements.

As the strategic value of emerging markets increase, pharmaceutical companies increase their R&D and manufacturing investments.

Bristol-Myers Squibb embarks on a multi-year journey to overcome the challenges of serialization and reap the benefits.

INTERPOL and 29 of the world’s largest pharmaceutical companies have joined forces in an initiative to battle counterfeit drugs.
As the strategic value of emerging markets increase, pharmaceutical companies increase their R&D and manufacturing investments.

Equipment purchased today for the packaging line should be serialization-ready in preparation for upcoming requirements.

Medication safety and efficacy depend on maintaining products at the proper temperature.

Protecting patients from counterfeit medicines is a pressing issue facing governments and the pharmaceutical industry.

Advances in data loggers and radio-frequency identification tags help meet the increasing need for managing the pharmaceutical cold chain.

As the battle against counterfeits gains momentum, PharmTech speaks to Mark Davison, CEO of Blue Sphere Health, and Craig Stobie, global life sciences sector manager at Domino Printing Sciences, about the challenges involved.

In addition to globalisation, high financial rewards and low penalties for counterfeiters are contributing to the rise in fake medicines.

Adeline Siew PharmTech speaks to Lynne Byers and Brian Johnson about Rx-360's initiatives to protect patient safety.

Overt and covert packaging technologies have evolved to authenticate drugs and fight counterfeits.

An operation spanning 16 African countries and conducted by the World Customs Organization (WCO) in partnership with the Institute of Research against Counterfeit Medicines (IRACM) led to the seizure of more than 82 million doses of counterfeit medicines.

Import controls and risk strategies aim to promote quality and spur new drug development.

A report from the European Commission shows that fake pharmaceuticals were the top articles detained by European Union customs in 2011.