
By focusing on laying strong technology foundations, pharmaceutical businesses can make informed decisions, equip themselves with the capabilities to eliminate threats, and future-proof operations.

By focusing on laying strong technology foundations, pharmaceutical businesses can make informed decisions, equip themselves with the capabilities to eliminate threats, and future-proof operations.

Technology is making it easier to stop problems before they can affect patients and the bottom line.

The European Commission publishes regulations on mandatory packaging safety features to fight counterfeiting.

The authors present analysis of the state of control of intermediate identity and quality, based on analysis of recently submitted DMFs.

Supply-chain success is measured by how effectively new medications reach patients, and how swiftly manufacturers can react to internal and external changes.
The authors provide their perspectives on shipping validation.

The agency prepares a plan to implement new packaging safety features.

Real-time GPS technology, better IT connections, and more conservative, controlled shipping temperatures are improving the shipment of sensitive pharmaceuticals.

Siemens and ADENTS develop joint solution for drug serialization challenges.

Traceability and transparency will remain elusive if manufacturers continue to approach serialization projects on a case-by-case basis.

The Safe Chain Track and Trace System automates traceability for supply chain security.

CSafe partners with Lufthansa Cargo in dedicating a fleet of RKN containers to Cool/td-Active service offering.
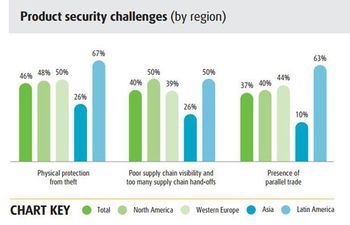
UPS’ 2015 “Pain in the Chain” survey suggests that pharma companies are getting better at product protection, cold chain and regulatory compliance, but need to improve cost control and planning for unexpected events. Lack of transparency and “too many handoffs” remain major challenges.

Virtual pilot programs examine scenarios that may occur while implementing serialization requirements for the US Drug Supply Chain Security Act.

Citing compliance issues, FDA extends deadline for product tracing information requirements for dispensers to March 1, 2016.
The global supply chain for bovine and porcine heparin and regulatory considerations are examined.

Rx-360 announced that Mark S. Paxton has been appointed as the new chief executive office of the international pharmaceutical supply chain consortium. This appointment follows the successful growth of Rx-360 since its foundation in 2009 in the aftermath of the adulterated heparin tragedy. Rx-360 has 300 volunteers from more than 100 institutions that are organized in 17 working groups with activities in the US, Europe, China, and India.

The challenge of achieving zero visible defects (i.e., particulates) in parenteral drugs will require a coordinated effort at all stages of the supply chain, particularly in the production and filling of primary containers.

FDA selected USDM Life Sciences, RC Partners, and The Clarion Group to develop and implement a DSCSA pilot project.

ReposiTrak signs agreement to pilot track and trace system in pharma.

GS1 standard tracks movement and status of goods, enabling better visibility, security and regulatory compliance.

UPS joins the Global Health Supply Chain Technical Assistance program to help secure the drug supply chain.

United Cargo signs global rental agreement with va-Q-tec for controlled transport of pharmaceuticals.

Virtual pilot programs that simulate scenarios can help the pharmaceutical industry address core issues in the implementation of serialization systems that comply with the US Drug Supply Chain Security Act.

Kate Kuhrt, senior director, Generics and Biotech, Thomson Reuters, spoke with Pharmaceutical Technology Europe about sourcing trends, supply chain challenges, the emerging market outlook, and how it affects European pharmaceutical manufacturers.