
Thomas LaVake, manager, worldwide environment, health &safety at Johnson & Johnson, provides a perspective of sustainability practices for the pharmaceutical industry.

Thomas LaVake, manager, worldwide environment, health &safety at Johnson & Johnson, provides a perspective of sustainability practices for the pharmaceutical industry.

Collaborative planning and execution among clinical research, clinical operations, and supply-chain managers are key elements in effectively managing an increasingly complex and global supply chain for clinical trial materials.

The US Food and Drug Administration issued a draft guidance for industry, Incorporation of Physical-Chemical Identifiers into Solid Oral Dosage Form Drug Products for Anticounterfeiting, on July 13.

The UK's Medicines and Healthcare Regulatory Agency (MHRA) has published the outcome of a consultation on measures to strengthen the country's drug supply chain.

During the past decade, big pharmaceutical companies have been making major efforts to restructure their manufacturing networks. In their haste to shed costs and assets, however, some may be adding risk to their product supply chains.

Consumer-care products, electronics, and select industrial firms comprise AMR Research's Supply Chain Top 25 for 2009, offering an opportunity for the pharmaceutical industry to examine best practices in supply-chain management.

As regulators work to curb counterfeiting, industry finds benefits to gaining granular data about the supply chain. This article contains bonus online-exclusive material.

In 2005, a small delegation (myself included) of the European Fine Chemicals Group (EFCG) met with the deputy head of the cabinet of Commissioner Kyprianou (the then Commissioner responsible for health and consumer protection). Our mission was simple - we were there to raise a red flag.

Today, approximately 1.5 million counterfeit medicine packs enter the legal supply chain each year - in other words, one pack in every 20000 is counterfeit.

The European Federation of Pharmaceutical Industries and Associations (EFPIA) announced a pilot program, to launch in Sweden later this year, that will focus on coding and identification solutions.

The United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA) seized nearly half a million pounds worth of counterfeit medicines on Mar. 26, 2009 in Middlesbrough, England, according to a MHRA press release.

Steps companies can take to help safeguard patients and the pharma supply chain.

After a year of increased attention on the pharmaceutical supply chain in Asia, what will be the region?s short? and long?term role?
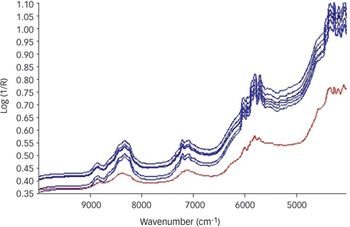
This month's expert examines the most appropriate technique for checking raw material quality. What technique would you recommend for quality checking of raw materials?

Last week, the US Food and Drug Administration issued a draft guidance for industry, Standards for Securing the Drug Supply Chain–Standardized Numerical Identification for Prescription Drug Packages, and launched a pilot program to help protect the pharmaceutical supply chain.

After a year of increased attention on the pharmaceutical supply chain in Asia, what will be the region's short- and long-term role? This article contains bonus online-exclusive material.

Creating better pharmaceutical and medical products with packaging partnerships.
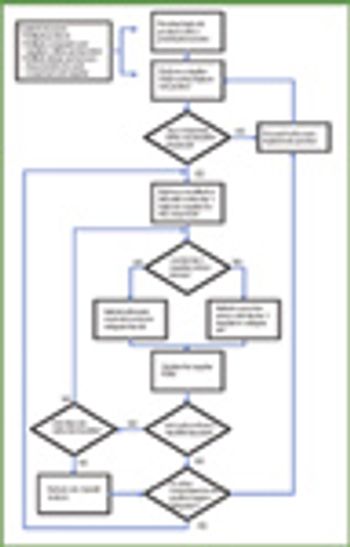
The growth and globalization of the pharmaceutical supply chain make risk assessment more important than ever for pharmaceutical manufacturers. The authors describe a program to identify, prioritize, mitigate, and communicate risks in manufacturer–supplier relationships.
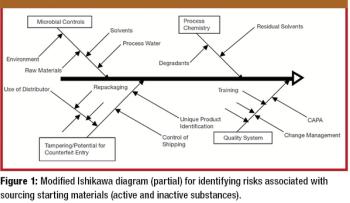
Regulatory bodies around the world are now revising legislation, regarding counterfeit medicines, good manufacturing and distribution practices, and risk management.
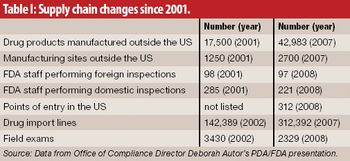
Pharmaceutical Technology has summarized recent statements by FDA officials on supply chain issues to provde the agency's most up-to-date views and expectations.
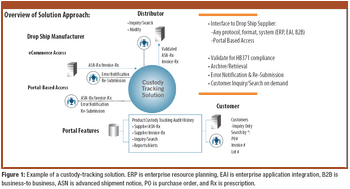
EPedigree, track-and-trace technologies, and other tools for optimizing supply-chain management are of increasing importance to the pharmaceutical industry. The author examines the current regulatory and legislative framework for ePedigree for finished drug products as well as proposals to require electronic statements for pharmaceutical ingredients.
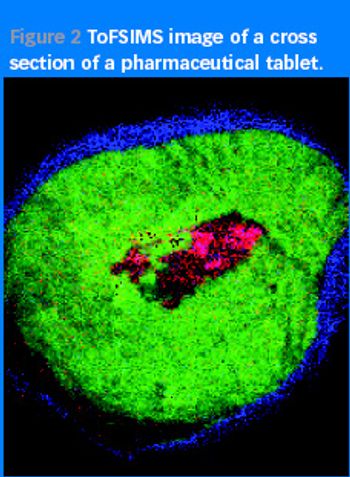
As counterfeiters become more cunning and technologically advanced, spotting their handiwork is increasingly difficult. Can surface analysis techniques be used to outwit them?

The House Committee on Energy and Commerce issued a revised discussion draft to the Food and Drug Administration Globalization Act of 2008.
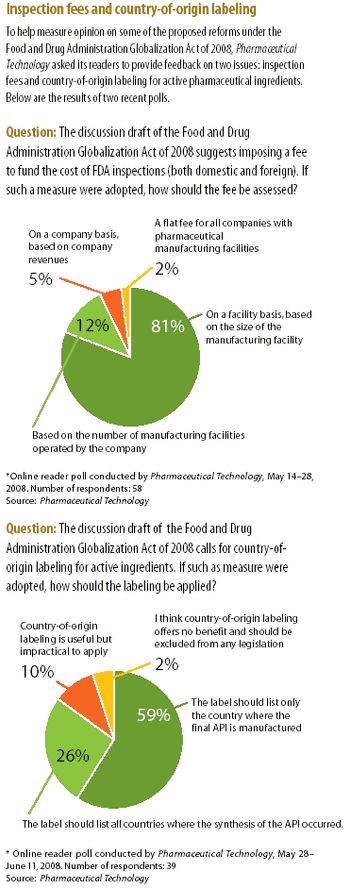
The high-profile case of contaminated heparin from a Chinese supplier has intensified the debate on the effectiveness of FDA's process for inspecting foreign drug-manufacturing facilities. The article examines proposed legislative and regulatory reforms and actions taken by the agency to improve drug-import safety.

How can any company be sure that the standards that suppliers might claim to operate, and might be able to demonstrate from time to time, are actually being practised all the time?