
As drug supply chain security is of paramount importance, could radio-frequency identification solutions provide the optimal solution for pharma companies?

As drug supply chain security is of paramount importance, could radio-frequency identification solutions provide the optimal solution for pharma companies?

With an increased focus on the supply chain security of non-cGMP intermediates, companies need to re-emphasize upstream manufacturing.

Discussing supply chain risk and security as a business led journey, Roddy Martin, chief digital strategist at TraceLink, explains the five stages of its evolution.

Proactive approaches that consider long-term supply chain security compliance are recommended to ensure companies stay on the right track.
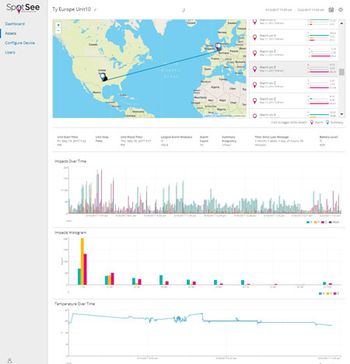
Considerations for monitoring impact, vibration, and temperature during package transportation and storage.

TraceLink will participate in the agency’s Pilot Project Program under the Drug Supply Chain Security Act.

A data exchange program between GE Healthcare and Amgen aims to improve biologics manufacturing through analysis of how raw materials affect the process.
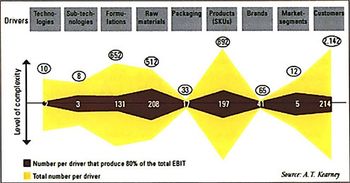
Novartis, BMS, and other companies are reducing complexity by thinning out product portfolios. Reducing complexity can reduce waste and improve responsiveness, but it must be done right.

Suppliers address complex supply chains for global biopharmaceutical manufacturing using disposable components.

GS1, a global supply-chain standards organization, launched a new messaging standard in collaboration with GS1 US to help meet the requirements of the US Drug Supply Chain Security Act (DSCSA) for salable returns of serialized prescription drugs.

The strategic partnership is expected to bring a new source of supply to providers, alleviating in particular the two-year shortage of metoprolol injection.

Recent advancements in cold-chain technology offer improved transporting and storing of temperature-sensitive pharmaceutical products.

A new initiative has been launched to help stimulate industry discussion on innovative tech for use across the drug supply chain.

TrakCel and WindMIL Therapeutics have formed a partnership to build a custom-configured cellular supply chain tracking and orchestration platform.

Lonza’s PyroTec PRO system combines robotic liquid-handling technology with an automation software module.

The companies will offer an IT platform that enhances the visibility and performance of the overall personalized medicine treatment process.

The plan details five goals to guide the development of Sentinel, a national electronic system for medical product safety surveillance established as part of the FDA Amendments Act of 2007, over the next five years.

Pharmaceutical Technology spoke with Steve Tallant, Systech, about the UniSecure solution, which won the 2018 CPhI Excellence in Pharma Award for supply chain, logistics, and distribution.

Active and intelligent packaging technologies benefit brand owners, caregivers, and patients.

Success depends on supplier communication and transparency, but it’s up to buyers to demand the right information and to look at the vendor’s overall business goals.

Adents' DispaX offers improved supply-chain traceability for pharmaceutical warehouses, wholesalers, distributors, and dispensers such as pharmacies and hospitals.

A coalition group from the UK's health sector has called on both the UK and EU to ensure the protection of patients and public health when considering the future relationship of the regions post-Brexit.

In preparation for the upcoming US Drug Supply Chain Security Act deadline, Metrics Contract Services has expanded serialization capabilities at its oral solid-dose commercial manufacturing site in Greenville, NC.

An ERP solution provides for the management of multiple business activities and traceability requirements resulting from regulations, customer demands, sourcing, and international business needs.

New FDA guidance offers a time buffer but increases supply-chain-management complexity, as active DSCSA enforcement begins.