
Advanced digital ledger technologies offer potential solutions, but are still several years away from practical use.

Advanced digital ledger technologies offer potential solutions, but are still several years away from practical use.

The contract development and manufacturing organization (CDMO) Aesica Pharmaceuticals has had a serialization program in place for the past five years, and recently installed capabilities for serialization at all its packaging facilities.

The agency will lead an international team to create a Supply Chain Security Toolkit of resources to educate the pharmaceutical and healthcare industries on supply chain vulnerabilities.

Intertek seeks to bring cost efficiencies to the pharmaceutical supply chain through confidential shared audits.

TruTags, the company’s silicon dioxide microtags, won Frost & Sullivan's 2017 North American Award for Technology Innovation.

The agency has pushed back by one year the deadline for compliance with DSCSA serialization requirements.

A survey from The Pistoia Alliance found that just under a quarter (22%) of life-science companies are already using or experimenting with blockchain, but industry collaboration on security and storage standards is needed.

In a new research conducted by SEA Vision and Zenith Technologies, 28% of respondents identified technology selection as the biggest challenge as the industry prepares to meet serialization deadlines.

The author shares his opinion on the challenges presented by the Internet of Things and what companies need to consider when choosing suitable architectures to manage serialization data.

The new UPS facility in Columbia will serve the growing pharmaceutical, biopharma, and medical device industry in Latin America.
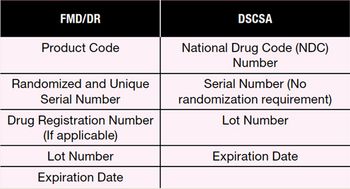
The author discusses upcoming serialization and transaction data collection regulations including the US Drug Supply Chain Security Act, the European Union Falsified Medicines Directive, and the EU Delegated Regulation.

Serialization and complementary authentication technologies are needed in order to meet DSCSA and FMD regulations.

A recent survey suggests that 36% of pharmaceutical companies and contract development and manufacturing companies have not started working on serialization, and that those who are working on it are focusing on basic compliance rather than potential long-term business benefits.

Due to weak penalties and lax enforcement, illicit pharmaceuticals continue to slip into the US, European and global supply chains.

The complex packaging and logistics required for personalized medicine pose significant challenges, but proactive planning can help ensure success.

Good project management, budgeting, planning, and clear documentation are the only ways to prevent overruns and project failure.

Pharmaceutical Technology spoke with Brad Pedrow and Rajesh Singh of Deloitte Consulting to discuss serialization implementation, and what to expect as the DSCSA deadline approaches.

igital tracking of overall equipment effectiveness can improve efficiency.

Uhlmann Packaging Systems joined the OPC Foundation’s Open Serialization Communication Standard (OPEN-SCS) Working Group.

Fisher BioServices will expand its CryoHub solution by co-locating it with the Cell and Gene Therapy Catapult manufacturing center for seamless supply chain management and to accelerate cell and gene therapy production.

FDA sent a warning letter to Lumis Global Pharmaceuticals Co. Ltd. detailing CGMP deficiencies regarding API repackaging, labeling, and misbranding.

Transparency between pharmaceutical companies and suppliers and risk assessment efforts are vital to effective supply chain practices.

As the November 2017 deadline nears, a surprising number of companies still don’t have a serialization plan in place. New programs aim to get them compliant in time.

The author discusses the results from TraceLink and Actionable Research's Global Drug Supply, Safety and Traceability Report.

Johnson & Johnson Supply Chain (JJSC) and the distributor AmerisourceBergen launched a four-week pilot program to test GS1’s EPCIS standards and to see how effectively data could be transferred between the two partners.