
Insulated shippers boost recyclable and renewable content.

Insulated shippers boost recyclable and renewable content.

Gore’s new flexible freeze containers are designed to protect high-value drug substances from container breakage or leakage.

TruTag Technologies, a provider of product identity solutions, has added mobile-phone authentication of solid oral-dosage form tablets to its product offerings.

As counterfeiting, API manufacturing issues, and illegal diversion increase vulnerability, could dispensers and even patients play a greater role in securing the pharma supply chain?
Air transport continues to be the most secure way to ship valuable therapies, but it is also the riskiest. Standards are helping to improve service quality.

The agency has issued a warning letter for cGMP violations at a drug manufacturing facility in Ahmedabad, India, that Baxter gained through its acquisition of Claris Injectables.

Findings from a survey show that some companies have not yet taken the necessary steps to ensure the continuity of medicine supply in the European Union after the United Kingdom’s departure.

Legislators look to widen access to medications for addiction treatment and overdose emergencies.

T&D’s new compact, battery-powered -75wf/nw line of thermocouple data loggers feature a temperature range of -199 to 1760 °C and free cloud storage.
Vendors are offering template-based models and direct data-filing services to help smaller companies meet imminent deadlines.

Bowe Systec has developed a modular stand-alone solution specifically designed to meet serialization requirements and that combines all the necessary functions in a single system. The new serialization solution will make its debut at Achema 2018.

Demand for direct-to-patient services is exploding. Michael Sweeney, senior director of patient-centric logistics at World Courier, discusses issues that the new model presents.

InstantGMP INV is a validated software for real-time material tracking and inventory control in biopharmaceutical manufacturing.

Shipping biopharmaceuticals in single-use containers requires a thorough understanding of the distribution cycle and potential transportation risks.

Increased use of single-use systems has led to a need to redefine safe, stable and integral systems for shipping biopharmaceuticals around the world. This article provides qualification data under international ASTM D4169 norms.

How warehouse execution systems can help in case of a drug recall.

Taking vendor product data at face value puts pharmaceutical manufacturers and their supply chain partners at risk.

Telstar reports that its Boreas, a new 86° ultra-low-temperature freezer, increases average performance by 20%.

The upcoming serialization deadline and the United Kingdom's departure from the European Union could result in supply bottlenecks.

Alan Kennedy, executive director of TEAM UP, shared perspectives on Poseidon and ocean transport with Pharmaceutical Technology.

Lower costs, fewer opportunities for temperature excursions, and a smaller carbon footprint are making ocean transport more attractive for pharmaceuticals. Poseidon, a new collaborative pharma initiative, seeks to leverage benefits.

The authors discuss expectations of regulators on the selection of drug substance regulatory starting materials (RSM) and the justification of their designation in the pharmaceutical supply chain, the scope of the RSMs' presentation required in regulatory filings, and how to mitigate and prepare for "push backs" in the event of a major objection to the sponsor's RSM designation.

The Multi-Chamber Blister System is a blister package that incorporates Amcor's SafeMix cold form laminate to transport vaccines and sensitive APIs without the need for cool storage.
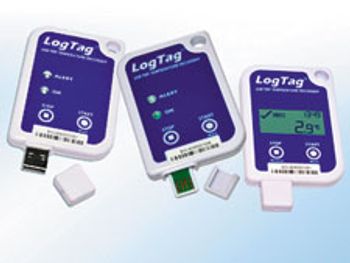
LogTag has added two new devices to its temperature data logger series.
Regulations and industry guidelines focus on ensuring excipient safety by specifying risk assessments and shared responsibility.