
Research groups supported by industry tackle the challenges of continuous crystallization and process analytical technology.

Cynthia A. Challener is a contributing editor to Pharmaceutical Technology.

Research groups supported by industry tackle the challenges of continuous crystallization and process analytical technology.

FDA steps up inspections of foreign API manufacturers, thanks to new legislation and changes in policy.

An integrated pilot plant tests heteronucleation and continuous crystallization.

Innovations for tablet tooling and presses improve quality and productivity.

New legislation and changes in policy at FDA are leading to better control of the API supply chain.

Innovative equipment, analytical techniques, software, and modeling systems are improving the tableting process.

Protecting workers, patients, and the environment requires advanced technologies.
Resolution technologies remain crucial for commercial-scale chiral API production.
Ligand-binding assays are fundamental to characterizing biosimilars.
Working with biological matrices and understanding the intended use are crucial.

Changing dynamics of the pharmaceutical industry are driving demand.

Dual sourcing is one of many possible solutions to securing the supply chain.

FDA approves treatments for new diseases and drugs that operate by new mechanisms.

On Oct. 7, 2014 at CPhI Worldwide in Paris, France, UBM Live announced the winners of the 11th annual CPhI Pharma Awards.
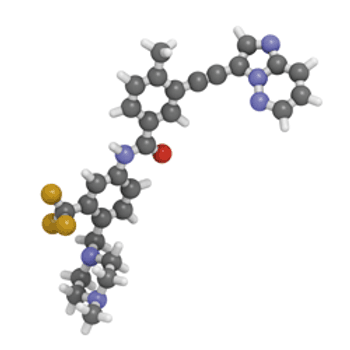
New practical approaches to the synthesis of complex heterocycles are reviewed.

Selected new technologies, products, and facilities from CPhI Worldwide 2014 are reviewed.

Advanced analytical methods are speeding up the targeted evaluation of potential viral contaminants.

Methods must be suitable at each development phase, robust, and effective on multiple platforms.
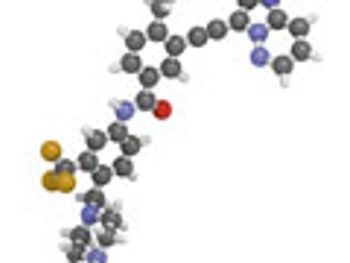
New practical approaches to the synthesis of complex heterocycles are reviewed.
The parenteral manufacturing industry is taking action to address particulate contamination issues.

Defining critical parameters and processing large quantities of data can be a challenge.

Supply chain security and quality group Rx-360 driven by patient safety.
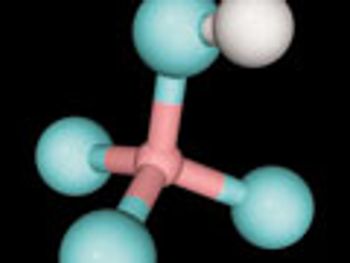
Fluorination can significantly impact the bioavailability of drug substances, however, safer fluorinating reagents and access to GMP fluorination capabilities remain challenges.

New investments, expansions, and company rebranding are discussed at CPhI.

The total market for biopharmaceuticals in 2013 was $36.8 billion according to Kalorama Information. Meanwhile, Frost & Sullivan estimates that the biosimilars market, which it pegged at just $1.2 billion in 2013, will grow to $23 billion in 2019, or more than 20-fold increase.

While advances in chemocatalysis for chiral synthesis continue apace, many companies have focused attention on the development of a wider array of enzymes that can be used under commercial manufacturing conditions for the selective conversion of various substrates into more complex intermediates.

The last 12 months have seen a number of major acquisitions in the contract manufacturing space.

Safer fluorinating reagents and access to GMP fluorination capabilities remain challenges in API synthesis.

Advances in solid and liquid formulation techniques are providing more options.