
Advanced drug delivery technologies can increase efficacy and safety, extend patent lives, and provide competitive differentiation for biopharmaceuticals.

Cynthia A. Challener is a contributing editor to Pharmaceutical Technology.

Advanced drug delivery technologies can increase efficacy and safety, extend patent lives, and provide competitive differentiation for biopharmaceuticals.
The commercial availability of an increasing diversity of enzymes has led to the growing use of biocatalysts for API synthesis.

The commercial availability of an increasing diversity of enzymes has led to the growing use of biocatalysts for API synthesis.

In order to ensure a safe drug supply chain, governments have implemented both legislated and voluntary programs designed to address problem areas.

Drug formulators are looking for new excipients to address solubility and bioavailability issues and extend patent protection, but there are hurdles to using novel, specialized ingredients.
Automated sample handling, advanced glycan analysis, and specially designed columns are help speed up confirmation of the biosimilarity.

Recent regulatory initiatives designed to secure the global pharmaceutical supply chain will directly impact the global supply chain and API manufacturers.
NMR analysis provides crucial structural information of synthesized glycans while LC-MS/MS is ideal for quantitation of free sugars in biological matrices.

For its use of innovative technology for the production of seasonal and pandemic influenza vaccines, the Novartis Holly Springs, North Carolina facility was the Overall Winner of the 2013 Facility of the Year Award issued by the International Society for Pharmaceutical Engineering (ISPE).

Use of a continuous-flow reaction made it possible to scale up a highly exothermic reaction for the production of a key Suzuki−Miyaura coupling reagent.

Measuring headspace gas composition and pressure allows sterile product manufacturers to simultaneously monitor important quality parameters rapidly and nondestructively.

The characteristics of tableted solid dosage drugs often depend on the granulation process, so selecting the most appropriate granulation technique and monitoring the process are crucial

Understanding the supply-chain challenge and coupling high-efficiency chromatographic techniques with information-rich detectors are leading to improvements in the management of extractables and leachables in parenteral drugs.

EMD Millipore and PharmaCell have entered into a collaboration to develop optimized large-scale expansion and harvest of HepaRG cells using bioreactor technology.

Synthesis of tetracycline derivatives with novel substituents has been challenging. Using an approach based on a Michael-Dieckmann reaction, new compounds with enhanced antibiotic properties are now being prepared on a large scale.

Pharmaceutical industry restructuring has created different strategies for drug manufacturers to consider in managing surplus laboratory and manufacturing assets.

For both small- and large-molecule applications, advances in mass spectrometry are leading to interest in this technique as an alternative to liquid chromatography.

New routes enable the efficient synthesis of enantiopure sulfinamides and structurally and sterically diverse P-chiral phosphine oxides.

Risk management guides decisions in facility design and operation for highly potent drugs.

Understanding the supply-chain challenge and coupling high-efficiency chromatographic techniques with information-rich detectors are leading to improvements in the management of extractables and leachables in parenteral drugs.

GE Healthcare's partnerships with iBio and Brazil's Bio-Manguinhos/Fiocruz for a new plant-based multipurpose biopharmaceutical and vaccine manufacturing facility move plant-based protein production to the next level.

Greater sophistication in 3D X-ray imaging technology raises its utility for QA/QC in manufacturing.

Since its inception in 2011, Fujifilm Diosynth Biotechnologies has added capabilities and single-use production capacity for mammalian-derived product manufacturing, upgraded development and pilot-scale microbial laboratories, and installed advanced analytical instrumentation.

Cocrystals can enable the formulation of solid dosage drugs, but the FDA's final guidelines have left concerns about how their use could impact development timelines, the drug product manufacturing process, and the intellectual property position of products containing cocrystals.

Serialization regulations in California and the EU come into effect in 2015 and 2016, and two US federal bills are moving through Congress. What are the implications for the pharmaceutical industry?

The benefits of single-use systems are being realized for downstream unit operations, including aseptic filling.

Until recently, glycan analysis has been a slow, labor-intensive process more widely used late in bioprocess development. New high-throughput methods are changing that.

Click chemistry has become a powerful tool in drug discovery, chemical biology, and proteomic applications.
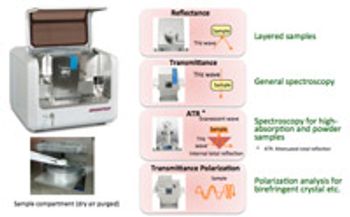
Now at a practical size and price, terahertz spectroscopy is advancing as a nondestructive method of analysis for solid forms and tablet coatings.

Aseptic connectors provide the flexibility and robustness needed for modern parenteral manufacturing operations.