
Following the United Kingdom’s decision to leave the European Union, there are two areas that demand attention-clinical trials and marketing authorization

Following the United Kingdom’s decision to leave the European Union, there are two areas that demand attention-clinical trials and marketing authorization

Horizon Pharma will acquire Raptor Pharmaceuticals with the transaction expected to close in the fourth quarter of 2016.

The companies launched Onduo, a joint venture focused on developing solutions to assist patients with Type 2 diabetes.

Results show the drug was effective at reducing the risk of uveitic flare or visual impairment.

A customer complaint revealed microbial contamination issues affecting lots with expiration dates in late 2018 and early 2019.

Mumbai, the location for its new Indian subsidiary, will also be the site for the company's Uniquity Global Conference on October 6, 2016.

Analysts examine the latest trends in finished dosage forms.

The company will be releasing its new 3C technology for omega 3 EPA and DHA at CPhI Worldwide.

The company will showcase its injectable grade pharmaceutical oils at CPhI Worldwide.

The company will display new products such as the Opadry QX, a tablet film coating.

FDA issued a warning letter to Lima & Pergher Industria e Comercio S/A for CGMP violations.

The Indian facility was cited for a range of quality and data integrity violations.

The agency published a guideline for the implementation of ICH Q3D.

Capsugel is expanding the facility to meet demand for micronization services for clinical and commercial manufacturing.

The department gave Moderna $8.2 million to accelerate development of an mRNA-based Zika vaccine.

Regulatory agencies meet to discuss approaches to the development of antibacterial agents.

Allergan acquired clinical-stage gene-therapy company, RetroSense Therapeutics, for $60 million.

The company established a dedicated research center in Bangalore, India for Amgen.

The company invested SEK 72 million ($9 million) to expand manufacturing capacity at its Karlskoga facility in Sweden.
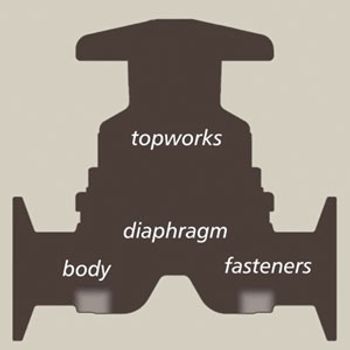
A process-specific preventative maintenance program improves productivity and reliability.

Aptar Pharma has signed a license agreement with BD to jointly develop and bring a new autoinjector to market.

Pricing of a lifesaving allergy treatment raises concerns.

The new facility is solely dedicated to offer extractables and leachables (E&L) testing services to the pharmaceutical and related industries.

The platform combines an expression system with equipment and process controls to enable rapid development and scale-up of robust titers.

CSafe completed the acquisition of Kallibox, a manufacturer of cold-chain packaging solutions.

Results from a small Phase Ib trial with Biogen’s aducanumab showed the drugs potential as an investigational treatment for patients with Alzheimer’s disease (AD). Researchers from the University of Zurich evaluated the efficacy of aducanumab in 165 patients with early-stage AD. At the conclusion of 54 weeks, the researchers reported seeing a dose-dependent reduction of amyloid-beta in patient’s brains compared to a placebo. Exploratory results indicate the drug may also slow clinical decline.

GE and Sealed Air extend their long-term collaboration to develop single-use films purposefully constructed for bioprocessing applications.

SpacePharma will give US researchers access to microgravity solutions to advance R&D projects.

The CordenPharma Chenôve, France manufacturing facility completed an FDA Inspection with no 483s reported.