
The National Institutes of Health (NIH) issued on July 7, 2009, final guidelines for human stem-cell research that widen the door to the cell lines that can be used if they meet certain ethical requirements.

The National Institutes of Health (NIH) issued on July 7, 2009, final guidelines for human stem-cell research that widen the door to the cell lines that can be used if they meet certain ethical requirements.

Also, Lundbeck acquired LifeHealth; FDA is seeking a director of its new tobacco regulation branch; Charles River Labs announces personnel changes; more...

The US Pharmacopeia is revising its monographs for four pharmaceutical excipients: propylene glycol, sorbitol solution, sorbitol sorbitan solution, and noncrystalllizing sorbitol solution.

Roche has officially withdrawn from the Pharmaceutical Research and Manufacturers Association (PhRMA) after being a member for 36 years.

Johnson & Johnson has agreed to pay $1.0 billion to acquire the assets and rights of the Alzheimer's immunotherapy program of the biopharmaceutical company Elan, form a new company with Elan based on the AIP program, and gain an 18.4% stake in Elan.

The Federal Coordinating Council for Comparative Effectiveness Research recommended that data infrastructure be the primary investment for the US Department of Health and Human Services's (HHS) comparative-effectiveness research (CER) funds.

As the pharmaceutical industry turns its efforts to biologic-based drugs and to more targeted delivery approaches, new tools are needed. Some recent developments are discussed.

Advances in micellar catalysis, solid-state chemistry, catalytic asymmetric synthesis, and function-oriented synthesis for natural products represent noteworthy developments in green chemistry that can be applied to the synthesis of active pharmaceutical ingredients.
The author provides a review of PAT and tools such as near infrared analysis that may facilitate the use of PAT in the biopharmaceutical sector.

On June 25, US Marshalls seized all drug products and ingredients at three facilities of Caraco Pharmaceutical Laboratories.

The UK's Medicines and Healthcare Regulatory Agency (MHRA) has published the outcome of a consultation on measures to strengthen the country's drug supply chain.

Also, Wyeth and Catalyst sign agreement; FDA seeks public opinion about tobacco regulation; Catalent appoints VP of quality and regulatory affairs; more...
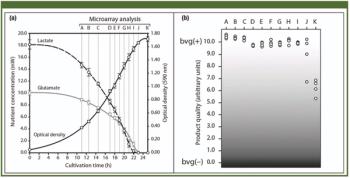
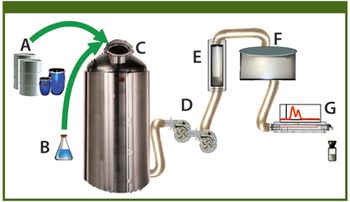
This case study describes the implementation of process analytical technology on the cultivation process step of a whole-cell vaccine against whooping cough disease.

Pan coating has been the preferred method of coating tablets for more than 20 years, but core coating is becoming more popular.

The Federal Trade Commission (FTC) last week issued an interim report that examined the effects of authorized generics on competition in the prescription drug market.

This week, the US Pharmacopeial Convention and the Vietnamese Pharmacopoeia Commission signed a memorandum of understanding that will help ensure the safety of Vietnamese medicines.
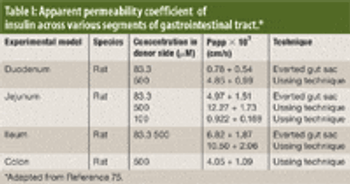
The authors review various oral drug delivery systems that have been explored to increase patient compliance for insulin.

While regulators begin to address nano-based drugs, industry should get its risk data ready.

Some GMP agents seem to find a way to squander time, money, and common sense.

Is it good policy to pay for bad behavior?

Traditional Chinese Medicine is widely used, but questions persist regarding its regulatory status.
The authors discuss how strategic outsourcing to contract manufacturing organizations that have technical and regulatory expertise can add further value during vaccine development.
Biopharmaceutical companies generally try to avoid paying royalties on novel technologies, including novel expression systems, in part because of the inability to predict revenue flow after a product is commercialized.

Patents are an important tool for protecting innovative products, uses or processes intended for commercialization.
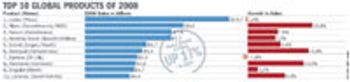
The world's top 50 pharmaceutical companies accounted for prescription drug sales of $558 billion (413 billion euro) in 2008.

Are manufacturers ready to deal with the economic and logistical challenges that accompany tailored therapeutics?

You are invited to Link In and Twitter with PTE!

Rare diseases represent an important area of unmet medical need.

Also, Adimab forms deals with Merck and Roche; Manhattan Pharmaceuticals' CEO and president steps down; more...

The Physician Payments Sunshine Act is still pending before the US Senate Committee on Finance, according to Jill Kozeny, communications director for Senator Chuck Grassley (R-IA).