
Generic-drug and specialty pharmaceutical manufacturer Watson Pharmaceuticals (Corona, CA) agreed to acquire the generic-drug company Arrow Group for $1.75 billion in cash and stock.

Generic-drug and specialty pharmaceutical manufacturer Watson Pharmaceuticals (Corona, CA) agreed to acquire the generic-drug company Arrow Group for $1.75 billion in cash and stock.

Former Senator Majority Leaders Howard Baker, Tom Daschle, and Bob Dole released a report this month, Crossing Our Line: Working Together to Reform the US Health System, which proposes four pillars of health reform.

Following the 2008 launch of the European Fine Chemicals Group (EFCG) Voluntary Guidelines (VGs) to promote supply-chain security, the group has now announced the official launch of an assessment template that will allow fine chemicals customers and suppliers to assess and implement this new set of recommendations.

The new commissioner of the US Food and Drug Administration is mapping plans for turning around an agency that has been demoralized, buffeted about in the press and has lost some of the trust of the American people.

Also, FDA debars clinical investigators; Jubilant Organosys forms deal with Endo Pharmaceuticals; AMRI makes changes to its India management team; more...

The Subcommittee on Health of the US House of Representatives Energy and Commerce Committee held hearings last week to discuss the findings of a report by the Federal Trade Commission (FTC) that examined the competitive effects for follow-on-biologics (FOBs).

Last week, the steering committee and expert working groups of the International Conference on Harmonization met in Yokohama, Japan, to discuss pending guidelines and other relevant issues.

Following the World Health Organization's declaration last week of an influenza pandemic, vaccine makers in Europe and China as well as world health agencies are stepping up efforts toward rapid development and approval of an effective vaccine.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the May 2009 edition from Bullard and Teledyne Tekmar.

The emergence of influenza A (H1N1) and the efforts to provide vaccines to the vulnerable are timely examples of biopharmaceuticals' continuing importance.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.

The UK's Medicines and Healthcare Products Regulatory Agency (MHRA) has published the results of its concept paper that sought comments regarding the review and consolidation of the UK medicines legislation.

Also, TorreyPines Therapeutics to liquidate assets and dissolve company; EU's competition services to examine Pfizer/Wyeth merger; Akorn appoints Raj Rai interim CEO; more...

Kemwell (Bangalore, Karnataka, India) plans to build a new biopharmaceutical manufacturing plant in Bangalore, India, in a strategic collaboration with Boehringer Ingelheim (BI, Ingelheim, Germany), according to a Kemwell press release.
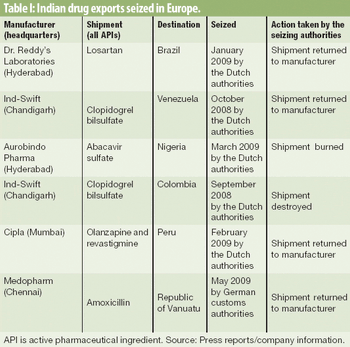
Patent infringement claims and a lack of clear global trade distribution routes may be unraveling the country's generic-drug export industry.

The US Government Accountability Office recommended that the US Food and Drug Administration draft a plan, including milestones, for developing its Sentinel system and ensuring the privacy and security of patients' healthcare data.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.

The US Food and Drug Administration released a new guidance for industry, Medication Guides-Adding a Toll-Free Number for Reporting Adverse Events, on June 8, 2009.

The chief operating officer (COO) of Mylan (Canonsburg, PA), one of the world's leading generic drug manufacturers, has condemned the launch of authorized generic drugs during 180-day exclusivity periods.

The European Commission's (EC) Directorate-General for Enterprise and Industry (EC-DG Enterprise) announced last week that it will not continue preparing a commission directive on good manufacturing practices (GMPs) for certain excipients.

The US biotechnology industry reached aggregate profitability for the first time ever in 2008, representing the only bright spot in an otherwise dismal year for biotechnology financing and performance.

Consumer-care products, electronics, and select industrial firms comprise AMR Research's Supply Chain Top 25 for 2009, offering an opportunity for the pharmaceutical industry to examine best practices in supply-chain management.

The United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA) is seeking opinions on a European Commission (EC) proposal that would allow drugmakers to disseminate certain information on prescription medicines directly to European Union patients.

Members of the House Committee on Energy and Commerce, Chair Emeritus John D. Dingell (D-MI), Chair Henry A. Waxman (D-CA), and Reps. Frank Pallone (D-NJ), Bart Stupak (D-MI), Diana DeGette (D-CO) and Betty Sutton (D-OH) released last week a discussion draft of the Food Safety Enhancement Act of 2009.

Also, WACKER expands Iowa facility; EMEA releases a Q&A document for PIPs; Metrics consolidates quality operations; more...

The International Serious Adverse Event Consortium (SAEC), a nonprofit partnership, has helped the US Food and Drug Administration gain significant understanding of the genetic basis for drug-induced liver injury, according a new FDA press release.

This week the US Food and Drug Administration released the final version of Guidance for Industry: Providing Regulatory Submissions in Electronic Format-Drug Establishment Registration and Drug Listing.

In two whistleblower suits filed in May 2009, the United States and 16 states alleged that Wyeth (Madison, NJ) knowingly failed to offer the government the same discounts it gave to private purchasers of its drugs, as Medicaid laws require.

A book guides readers through the regulatory requirements for computerized quality systems.

Compliance features help patients follow medication regimens correctly.