
Pharmaceutical Technology spoke to experts in the field of biopharmaceutical manufacturing to gain insights on top trends that are currently shaping the industry.


Pharmaceutical Technology spoke to experts in the field of biopharmaceutical manufacturing to gain insights on top trends that are currently shaping the industry.

Potentiometric titration systems from Metrohm can be customized for different applications.

Gyrolab CHO-HCP Kit 1 detects and quantifies host cell proteins from Chinese hamster ovary cells.

The countdown has begun for one of the biggest trade shows for the process industry.

While biotech will revolutionize our industry through new products and new reaction pathways, process analytics is the link between process automation and the laboratory sector, and water plays a key role as a valuable resource all over the world.

Waters announces it will introduce its new technology at the 63rd Society of Mass Spectrometry conference.

Shimadzu’s Nexera Method Scouting system provides an outstanding platform for efficient HPLC method development and implementation. It is based on the Nexera X2 next-generation ultra-high performance liquid chromatograph, an all-round LC system for ultra-high-speed analysis.

Medicago's new production facility will make plant-based vaccines and therapeutics.

This article looks at data gathered from several studies of a widely marketed chlorobutyl rubber formulation used for vial stoppers and prefilled syringes.

Traditional sterility testing methods can take 14 days or longer to complete, so a growing number of pharmaceutical manufacturers and quality control
The rapid testing of biologic raw materials can lead to greater efficiency.
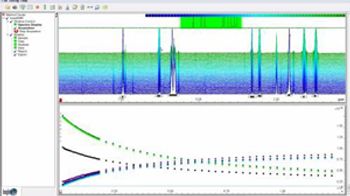
Bruker's InsightMR software for on-line NMR analysis enables real-time data analysis and acquisition control for process monitoring.

Shimadzu’s Nexera MP UHPLC Front End system for LC/MS is perfect for LC/MS analysis conducted in pharmacokinetics and synthesis stages in drug detection processes.

In this article, industry experts discuss critical analyses for demonstrating biosimilarity.

USP announces an implementation date of Jan. 1, 2018 for General Chapters Elemental Impurities-Limits and Elemental Contaminants in Dietary Supplements.

The agency outlines recommendations for the development and submission of near infrared analytical procedures.

The commission adopts 24 new and 72 revised texts for inclusion in the European Pharmacopoeia.

The BallProbe from MarqMetrix is an optical sampling interface for inline, real-time measurements.

NSF's consensus-based standard incorporates multiple regulatory and industry requirements into a single rigorous standard for the manufacturing and distribution of pharmaceutical excipients.

Analytical technologies must accurately identify and measure the critical material attributes of APIs and excipients.

PAT has aided formulation, both pre-and post-filing, by reducing costs and time frames.

Assessing risk factors is key to implementing the new ICH Q3D guidelines for elemental impurities.

The pharmaceutical manufacturing platforms of fluid-bed granulation and milling are widely used to modify particle size. However, the adoption of process analytical technology to monitor and control these processes is difficult because of their dynamic nature. This study examines the efficacy of a particle characterizing technology to capture particle images under dynamic conditions and to calculate particle size distribution data both in-line and at-line during fluid-bed granulation and milling.

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.

The all-synthetic 3M Emphaze AEX Hybrid Purifier contains both an anion-exchange nonwoven media and a fine-particle, bioburden reduction membrane.