
The choice of dilution schemes can minimize solution preparation errors.


The choice of dilution schemes can minimize solution preparation errors.

Analytical and procedural deficiencies result in FDA warning letter for Novacyl Wuxi Pharmaceutical.

Symbion QT chemometrics software provides Parametric Data Cleaning to handle data compromised by excessive noise or other artifacts.

Advanced analytical methods are speeding up the targeted evaluation of potential viral contaminants.

Methods must be suitable at each development phase, robust, and effective on multiple platforms.

Analytical procedures and method validation should be developed with a structured and rigorous approach.

The authors present a validation study of an analytical method for the simultaneous determination of nine human cytokines in human K2-EDTA plasma.

The authors describe a rapid, single-point calibration approach for ICP–MS analysis of raw materials used in drug product manufacturing.

Industry players form Allotrope Foundation to solve analytical data management problems.

Recent technology introductions demonstrate that accuracy, efficiency, and usability are top of mind for pharmaceutical industry professionals for analytical instruments.

MedImmune enters into an agreement to acquire the image analysis and data mining company.

A range of balance models cover a weighing capacity from 60 to 8200 grams.

A risk-based approach is recommended for analytical method comparability for HPLC assay and impurities methods.

Data analytic strategies can help companies capitalize on personalized medicine.
Review challenges in the use of normality testing situations and recommendations on how to assess data distributions in the pharmaceutical development manufacturing environment
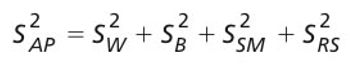
A risk-based guard band surrounds a specification limit and is derived from the uncertainty of the reportable value of the analytical procedure, which includes the uncertainty in the reference standard. The author discusses requirements for generating a reportable value and calculating the associated measurement uncertainty.

Transcat's Cost, Control, and Compliance (C3) software platform is designed to improve asset tracking and process management for metrology instrumentation.
Febuxostat is a novel, non-purine, selective inhibitor of xanthine oxidase for hyperuricemia in patients with gout. It is the first promising substitute for allopurinol in 40 years. Various synthetic routes to febuxostat, as well as polymorphic forms and impurities of the drug, are reported in the literature. The authors have also identified several impurities that result from the synthesis of febuxostat. This article describes the identification and control of all isomeric, carryover, and byproduct impurities of febuxostat and its intermediates.
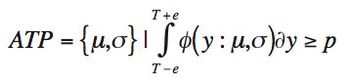
The quality-by-design principles used to control process variability are equally important to measurement systems because process variability includes contributions from measurement system variability. The authors use real-life examples from drug development projects to outline how an understanding of chromatographic measurement system variability might be achieved.

Changing regulations are impacting the identification and monitoring of variable materials in excipients.

The latest revisions to the USP General Chapters <41> Balances and <1251> Weighing on an Analytical Balance aim to ensure weighing accuracy and eliminate unnecessary overtesting by simplifying previous descriptions and reflecting current state-of-the-art weighing practices.

Developments in chromatography column technology to deliver greater efficiency, speed and inertness benefit the drug development process from discovery to manufacturing and quality control.

A diverse range of particle sizing solutions is available, from techniques for the characterization of complex formulations through to online PAT tools for real-time measurement.

Contract research, development, and manufacturing organizations invested in new facilities and technologies in 2014 for analytical testing, as well as small- and large-molecule drug development.

Experts from contract testing laboratories and service organizations shared their perceptions of analytical testing advances, and challenges still ahead.