
Scale-down modeling is instrumental in supporting the development of downstream biopharma manufacturing processes.

Scale-down modeling is instrumental in supporting the development of downstream biopharma manufacturing processes.
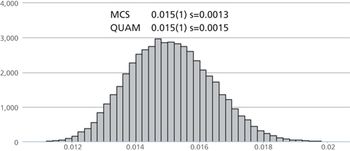
Performing a measurement uncertainty calculation is often seen as problematic.

Today’s analytical laboratory equipment reflects the realities of downsizing, outsourcing, and the need for speed.

Keith Moore, vice-president of analytical services, Metrics Contract Services discusses gains use in dissolution testing.
Time and sensitivity are essential for analytical technologies in all phases of biopharma development.

Today’s analytical laboratory equipment reflects the realities of downsizing, outsourcing, and the need for speed.

The European Directorate for the Quality of Medicines & Healthcare announces the publication of a chemometric methods chapter in the European Pharmacopoeia.

The European Pharmacopoeia rewrites in its general chapter on Raman spectroscopy.

FDA issued a warning letter to Zhejiang Hisun Pharmaceutical Co., Ltd., as a result of inspections that took place on March 2–7, 2015 at the Taizhou City, Zhejiang Province, API manufacturing facility.

Sensaphone's monitoring system provides low-cost, 24/7 remote monitoring of unattended freezers and coolers.

An ultraviolet light (UV) spectrophotometer system from Hanson Research automatically handles samples for pharmaceutical dissolution and diffusion testing.

The Prominence-i LC-2030 LT is a new model of Shimadzu’s i-Series integrated high-performance liquid chromatograph and ultra high-performance liquid chromatograph systems.

Mass Spec Lab, a privately owned analytical company, announced its official launch on Dec. 28, 2015 in Southern California.

Shimadzu’s Nexera MP UHPLC Front End system for LC/MS is perfect for LC/MS analysis conducted in pharmacokinetics and synthesis stages in drug detection processes. read more

Analytical methods and functional assays are used to compare molecules and relate characteristics to quality attributes.

KnowItAll spectroscopy software from Bio-Rad Laboratories corrects differences in spectra data.

Charles River offers the Endosafe nexgen-PTS endotoxin testing system.

Agilent Technologies and Thermo Fisher Scientific exchange instrument control drivers and software support.

Copley Scientific’s new Vertical Diffusion Cell Test System Model HDT 1000 comprises a compact unit with 10 test stations that enables the precise control of test conditions.

The Nexera UC Unified Chromatography System from Shimadzu Scientific Instruments was recognized with an R&D100 award.
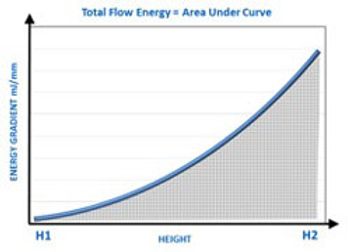
Dynamic powder testing and measurement of bulk powder properties can complement shear cell testing to identify the causes of poor hopper performance in solid-dosage drug manufacturing.

Mettler Toledo Thornton’s 7000RMS delivers on-line, real-time, continuous measurement of microbes and inert particles in purified pharmaceutical waters.

Problems in an induction-sealing process, such as untorqued or crooked caps, can be identified and corrected in real time using dynamic thermal imaging.

Shimadzu’s new LCMS-8060 is designed to push the limits of LC/MS/MS quantitation for applications requiring the highest sensitivity and robustness while delivering a meaningful solution for routine LC/MS/MS analyses.

Shimadzu and Hanson Research partner on pharmaceutical testing instrument integration.